Biological Importance of the Indole Nucleus in Recent Years a Comprehensive Review
Biological activities of indole
Antiviral activity
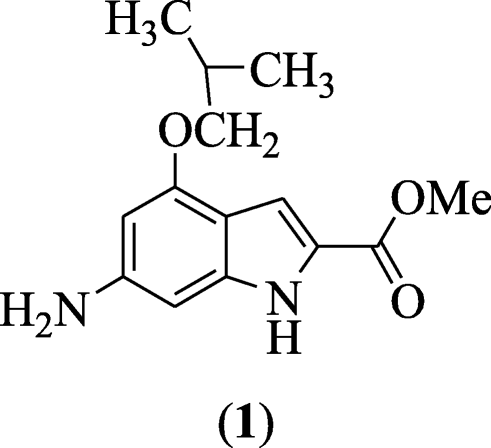
6-Amino-iv-substitutedalkyl-1H-indole-two-substitutedcarboxylate derivatives were prepared and reported equally antiviral agents by Xue et al. In all tested compounds, compound methyl 6-amino-four-isobutoxy-1H-indole-two-carboxylate (1) showed inhibitory activity against flu A with IC50 = 7.53 μmol/L and the highest selectivity index (SI) value 17.one to CoxB3 virus [4].
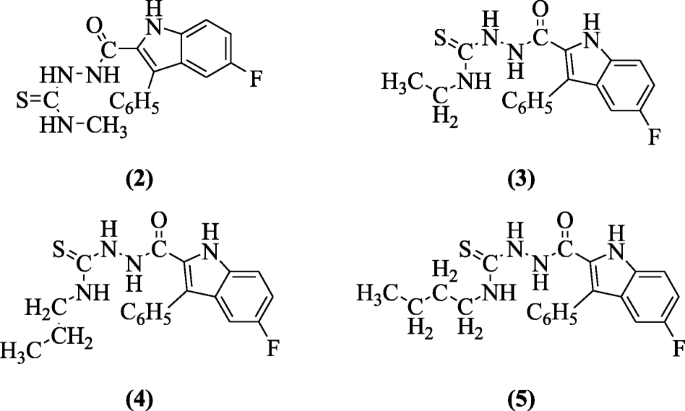
four-Alkyl-i-(5-fluoro-3-phenyl-aneH-indole-2-carbonyl)thiosemicarbazide derivatives of indole were prepared and investigated in vitro for antiviral activity in a broad range of ribonucleic acid (RNA) and deoxyribonucleic acrid (DNA) viruses by Cihan-Üstündag et al. Compounds 1-(five-fluoro-3-phenyl-oneH-indole-2-carbonyl)-iv-methylthiosemicarbazide (2), 4-ethyl-i-(5-fluoro-3-phenyl-1H-indole-ii-carbonyl)thiosemicarbazide (3), 1-(5-fluoro-iii-phenyl-1H-indole-two-carbonyl)-4-propylthiosemicarbazide (4), and 4-butyl-1-(5-fluoro-three-phenyl-1H-indole-2-carbonyl)thiosemicarbazide (5) are stiff antiviral agents with IC50 values ranging from 0.4 to two.1 μg/mL against Coxsackie B4 virus [5].
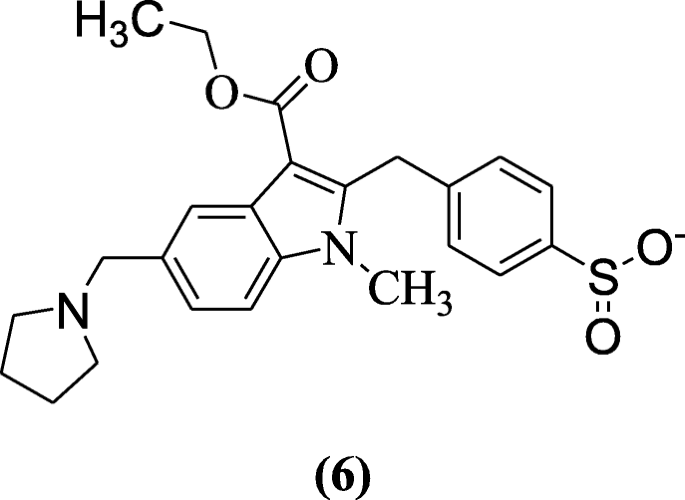
Ethyl oneH-indole-3-carboxylates too showed anti-viral activity in Huh-vii.5 cells explained by Sellitto et al. Compound iv-((3-(ethoxycarbonyl)-ane-methyl-5-(pyrrolidin-1-ylmethyl)-1H-indol-2-yl)methyl)benzenesulfinate (vi) was the most agile compound at depression concentration against hepatitis C virus (HCV) [half dozen].
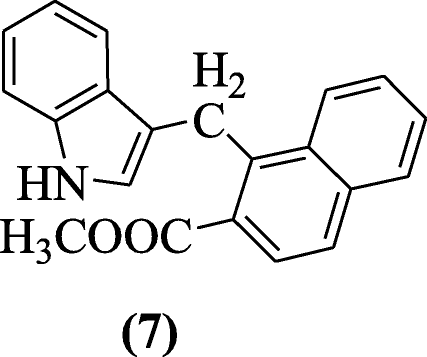
Giampieri et al. elaborated reaction of indoles and two-naphthols through Mannich bases and tested against different viruses and compound methyl i-((1H-indol-iii-yl)methyl)-2-naphthoate (seven) showed significant action confronting Yellow Fever Virus (YFV), Bovine viral diarrhea virus (BVDV), Human being immunodeficiency virus-1 (HIV-i), and Respiratory syncytial virus (RSV) [7].
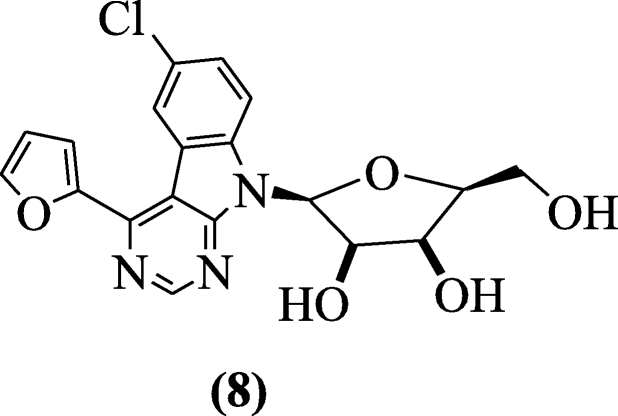
Pyrimidine-derived indole ribonucleosides (2S,3R,fourS,5South)-two-(6-chloro-4-(furan-2-yl)-9H-pyrimido[4,5-b]indol-9-yl)-5-(hydroxymethyl)-tetrahydrofuran-3,4-diols were synthesized and tested for in vitro antiproliferative (HL-threescore cervical carcinoma HeLaS3, T-lymphoblastic leukemia human jail cell line CCRF–CEM andpromyelocyticleukemia) and antiviral activity (Dengue virus and anti-hepatitis C virus) past Tichy et al. Chemical compound (2S,iiiR,4Southward,fiveS)-ii-(6-chloro-four-(furan-2-yl)-9H-pyrimido[four,5-b]indol-ix-yl)-five-(hydroxymethyl)-tetrahydrofuran-3,4-diol (eight) exhibited the notable cytotoxicity in HepG2 cells and THP-one with ICl of 0.175 and 1.565 μM [8].
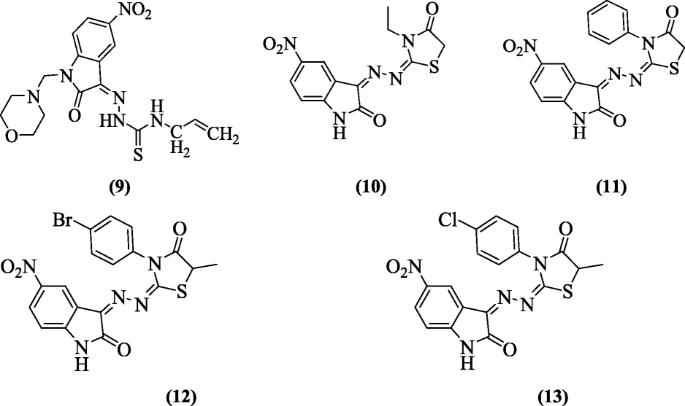
5-Nitro-3-[(5-nonsubstituted/methyl-4-thiazolidinone-two-ylidene) hydrazono]-1H-ii-indolinones were prepared and tested for antiviral activities by Terzioğlu et al. Compounds (Z)-four-allyl-1-(ane-(morpholinomethyl)-5-nitro-two-oxoindolin-3-ylidene)thiosemicarbazide (9), (threeZ,3E)-3-(ii-(3-ethyl-4-oxothiazolidin-2-ylidene)hydrazono)-five-nitroindolin-2-one (10), (3Z,3Eastward)-5-nitro-3-(2-(4-oxo-three-phenylthiazolidin-two-ylidene)hydrazono)indolin-2-one (xi), (3Z,3E)-3-(ii-(3-(four-bromophenyl)-v-methyl-iv-oxothiazolidin-2-ylidene)hydrazono)-5-nitroindolin-ii-ane (12) and (3Z,3E)-3-(ii-(3-(4-chlorophenyl)-5-methyl-4-oxothiazolidin-2-ylidene)hydrazono)-5-nitroindolin-2-ane (13) prevented the development of bovine viral diarrhea virus in cells [9].
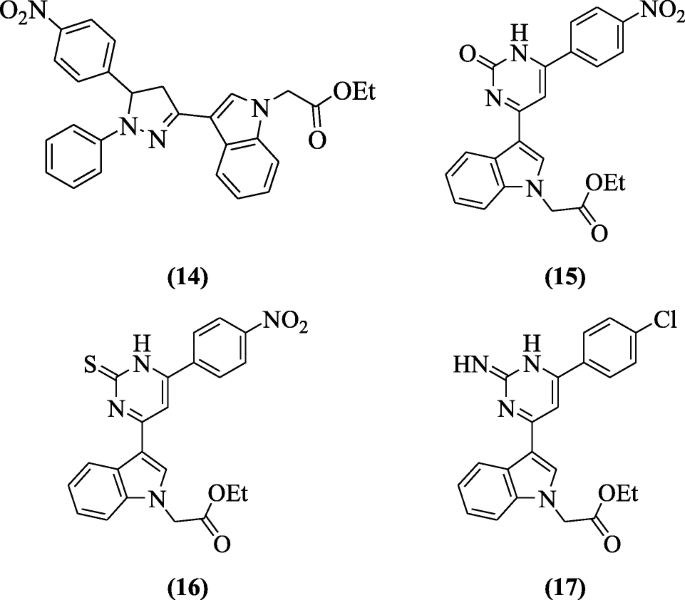
vii-Ethoxy-1-methyl-4, 9-dihydro-threeH-pyrido [3, four-b]indole derivatives were reported as anti-Canker Simplex virus-i(HSV-1) compounds past El-sawy et al. and derivatives ethyl ii-(3-(5-(4-nitrophenyl)-1-phenyl-four,5-dihydro-1H-pyrazol-3-yl)-1H-indol-one-yl)acetate (14), ethyl ii-(3-(6-(4-nitrophenyl)-2-oxo-1,two-dihydropyrimidin-4-yl)-oneH-indol-1-yl)acetate (15), ethyl ii-(3-(six-(4-nitrophenyl)-ii-thioxo-one,2-dihydropyrimidin-four-yl)-1H-indol-1-yl)acetate (xvi) and ethyl 2-(three-(6-(4-chlorophenyl)-2-imino-ane,2-dihydropyrimidin-4-yl)-aneH-indol-1-yl)acetate (17) possessed considerable antiviral action with IC50 ranged between 5 and vi μg/ml and substantial therapeutic indices (TI) of 80 and 83 were recorded [x].
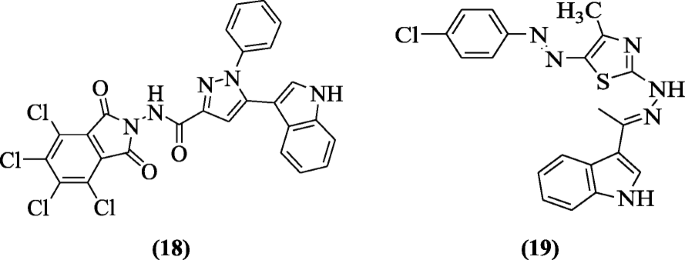
1,3-Thiazole and ane,2,four-triazolo[3,4-b]i,3,4-thiadiazine containing indole nucleus derivatives were prepared and checked for their antiviral activity against HSV-1(herpes simplex type1) by Abdel-gawad et al. Compounds 5-(1H-indol-3-yl)-one-phenyl-N-(4,5,half-dozen,7-tetrachloro-i,3-dioxoisoindolin-2-yl)-1H-pyrazole-three-carboxamide (eighteen) and two-(2-((E)-1-(aneH-indol-3-yl)ethylidene)hydrazinyl)-v-((East)-(four-chlorophenyl)diazenyl)-4-methylthiazole (xix) showed all-time action against HSV-1 [11].
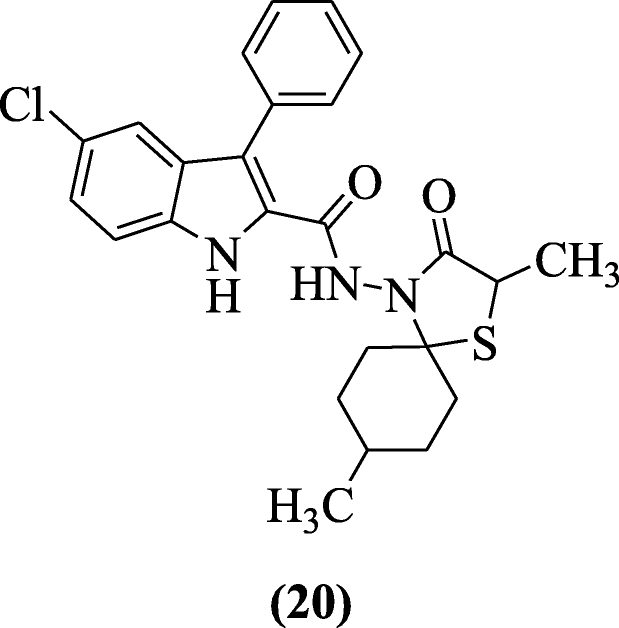
Indole-based spirothiazolidinones besides have antiviral activity as discussed by Cihan-Üstündağ et al. Compounds presented inhibitory action in Vero cells confronting yellow fever and Punta Toro virus. The range of ICfifty values was 1.nine–12 μM. Compound v-chloro-Due north-((2Southward,5Southward, 8R)-2,8-dimethyl-iii-oxo-1-thia-4-azaspiro[four.five]decan-iv-yl)-3-phenyl-iH-indole-2-carboxamide (20) was the most active [12].
Anti-inflammatory and analgesic activities
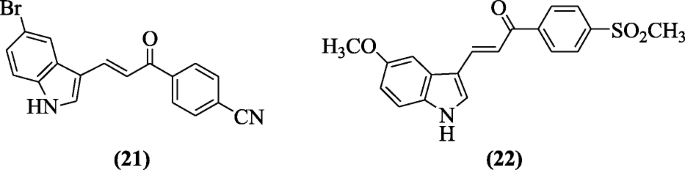
Indole-based chalcone derivatives reported as COX-i and COX-2 inhibitor by Ozdemir et al. Compound three-(5-Bromo-1H-indol-3-yl)-1-(iv-cyanophenyl)prop-2-en-1-one (21) and compound iii-(5-methoxy-aneH-indol-3-yl)-1-(4-(methylsulfonyl)phenyl)prop-2-en-one-i (22) were constitute to demonstrate a significant activity [13].
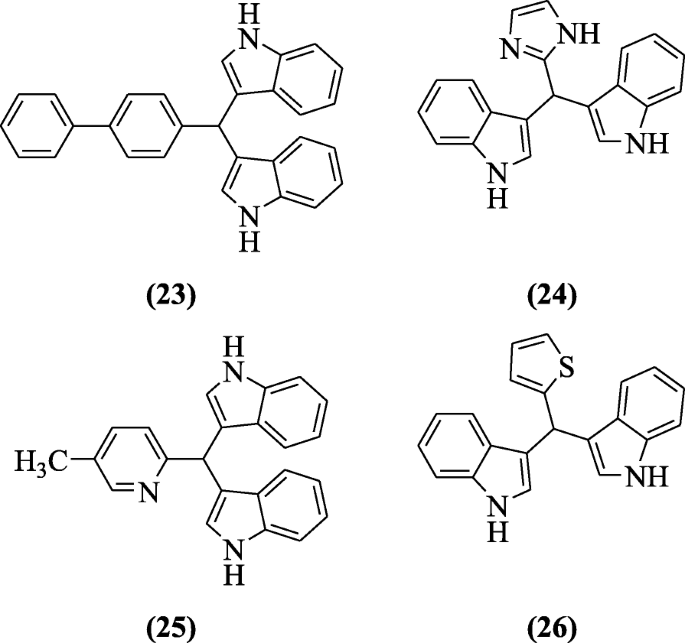
Sarva et al. carried out solvent-free reaction in microwave betwixt indole and substituted aldehydes. The product, bis(indolyl)methane is bioactive. The anti-inflammatory action was shown by nigh of the compounds but compounds 3,3'-([1,1'-biphenyl]-4-ylmethylene)bis(oneH-indole) (23), iii,three'-((1H-imidazol-2-yl)methylene)bis(1H-indole) (24), 3,three'-((5-methylpyridin-2-yl)methylene)bis(iH-indole) (25) and iii,3'-(thiophen-two-ylmethylene)bis(aneH-indole) (26) were the almost stiff [xiv].
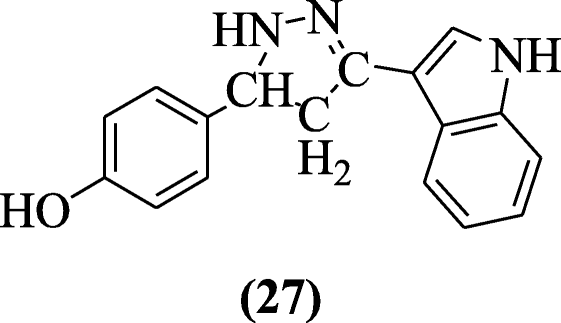
Anti-inflammatory activities of chalcones of indole were elaborated by Rani et al. confronting carrageenan-induced edema in albino rats. The most constructive compound of this serial was institute to be 4-(3-(1H- indol-iii-yl)-iv, v-dihydro-oneH-pyrazol-5-yl) phenol (27) [15].
Indole containing isoxazole derivatives were reported by Pedada et al. as sPLA2 inhibitory agents. Chemical compound N-((3-(four-fluoro-3-(trifluoromethyl) phenyl) isoxazol-five-yl) methyl) (v-methyl-1H-indol-3-yl) methanamine hydrochloride (28) showed meaning sPLA2 inhibition activity that is comparable or more to ursolic acrid (positive control) [xvi].
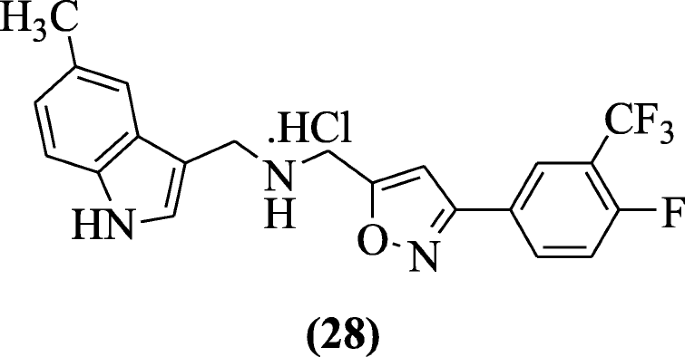
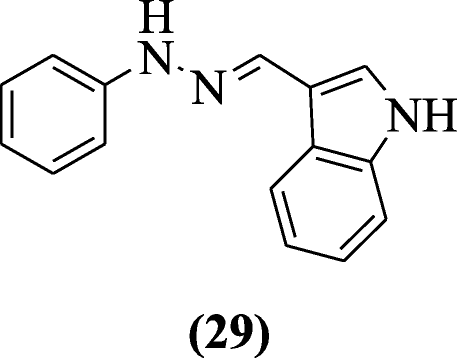
Reactive oxygen species (ROS) generation and nitric oxide release induced through lipopolysaccharide were inhibited free radicals HMPH (1-[(1H-indol-3-yl)methylene]-2-phenylhydrazine scavenged) in RAW cells without any cytotoxicity explained by Misra et al. In all tested compounds, compound (Due east)-1-((1H-indol-iii-yl)methylene)-ii-phenylhydrazine (29) showed significant activity [17].
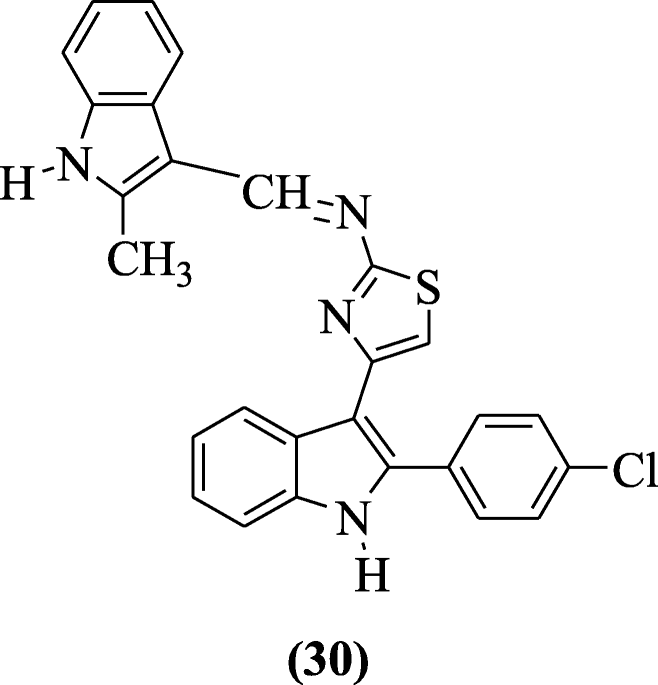
3-(2'-Substituted indolidene aminothiazol-4'-yl)-2-(4-chlorophenyl) indoles derivatives were reported as analgesic and anti-inflammatory agents by Singh et al. Compound (Due east)-four-(ii-(4-chlorophenyl)-1H-indol-3-yl)-N-((2-methyl-1H-indol-3-yl)methylene)thiazol-2-amine (30) showed the ameliorate effect every bit anti-inflammatory and analgesic amanuensis [18].
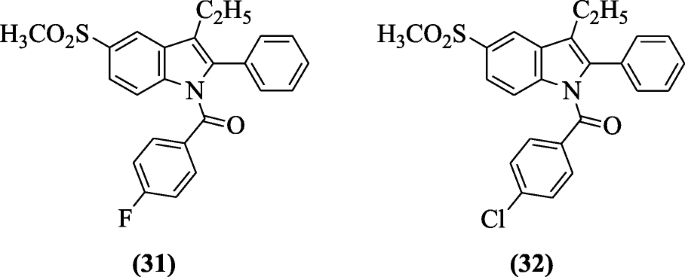
(3-Ethyl-five-(methylsulfonyl)-ii-phenyl-oneH-indol-1-yl) substitutedphenyl methanones were prepared and tested for their anti-inflammatory activity confronting COX enzymes. For in vitro studies, all of the tested compounds, especially compounds incorporating So2Me moiety as a COX-ii pharmacophoric characteristic, showed preferential inhibitory activity confronting COX-two over COX-1(SI = 4.02 to 65.71) compared with indomethacin (SI = 0.079). Whereas in vivo anti-inflammatory activity was proficient for the compound having And so2Me moiety and compounds (3-ethyl-5-(methylsulfonyl)-2-phenyl-aneH-indol-1-yl)(four-fluorophenyl)methanone (31) and (4-chlorophenyl)(3-ethyl-5-(methylsulfonyl)-2-phenyl-1H-indol-i-yl)methanone (32) were more potent than indomethacin. The presence of a carbonyl moiety as a spacer instead of methylene resulted in an increase in the anti-inflammatory activity [19]
three-Methyl-2-phenyl-ane-substituted-indole derivatives were synthesized and investigated for anti-inflammatory (in vitro and in vivo) and analgesic activities by Abdellatif et al. Derivatives (3-methyl-5-(methylsulfonyl)-two-phenyl-1H-indol-1-yl)(phenyl)methanone (33), (4-chlorophenyl)(3-methyl-5-(methylsulfonyl)-2-phenyl-1H-indol-1-yl)methanone (34), and 1-benzyl-three-methyl-five-(methylsulfonyl)-2-phenyl-1H-indole (35) showed the highest anti-inflammatory (in vitro and in vivo) and analgesic activities. The results of molecular docking studies were in agreement with that obtained from the in vitro COX inhibition assays [20].
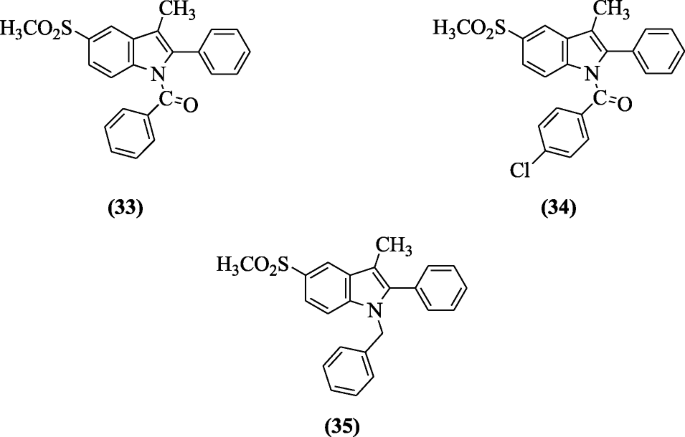
3-(two-Aminopyrimidin-4-yl) indoles were prepared and investigated for their ulcerogenic, anti-inflammatory, and analgesic activities past Chavan et al. All the synthesized compounds showed alike results with indomethacin. Among the tested compounds, compounds iv-(two-amino-6-(2-(4-chlorophenyl)-1H-indol-3-yl)pyrimidin-4-yl)phenol (36) and 4-(4-aminophenyl)-6-(2-(4-chlorophenyl)-1H-indol-3-yl)pyrimidin-two-amine (37) showed 87.iv and 88.2% inflammation inhibition using mitt edema, 78.5 and 76.6% inhibition of acerb acid-induced writhings [21].
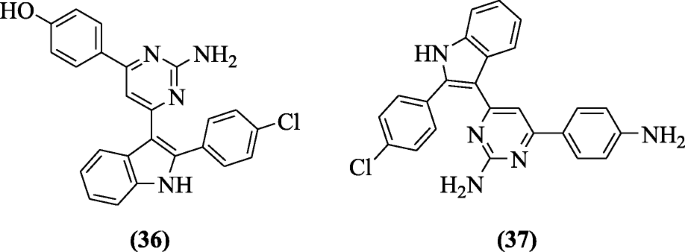
Some new derivatives of 3-(2'-carboxy-5'-mehoxyindol-3'-yl)-v-(substituted phenyl)-2-isoxazolines have been prepared with less CVS and CNS activities simply practiced anti-inflammatory activity by Prajapati et al. All the compounds were investigated for anti-inflammatory activity and compound five-methoxy-iii-(3-phenylacryloyl)-iH-indole-2-carboxylic acid (38) showed maximum activity (64.20%) at fifty mg/kg [22].
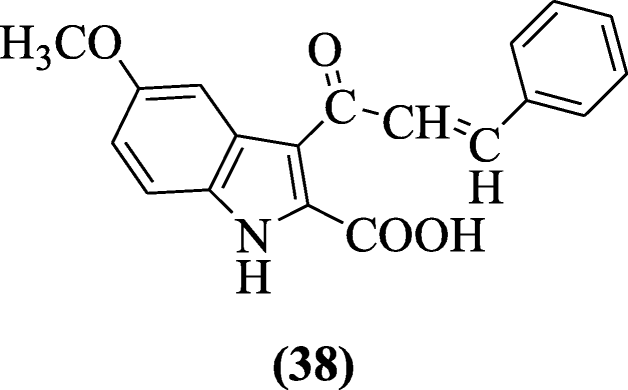
i, viii-Dihydro-1-aryl-8-alkyl pyrazolo(3,4-b)indoles were synthesized by Mandour et al. that showed remarkable anticonvulsant, analgesic, and anti-inflammatory activities in comparison to diazepam, flufenamic acid, and indomethacin equally positive controls. Among the tested compounds, the most potent compounds were institute to be (6-chlorocyclohexa-2,4-dienyl)(ane-(2,iv,six-trichlorophenyl)pyrazolo[3,4-b]indol-viii(1H)-yl)methanone (39) and (half dozen-chlorocyclohexa-2,four-dienyl)(1-phenylpyrazolo[3,4-b]indol-8(1H)-yl)methanone (40) with percentage inflammation inhibition 85 and 79% (100 mg kg–1) and 70 and 64% ( 50 mg kg–ane) respectively [23].
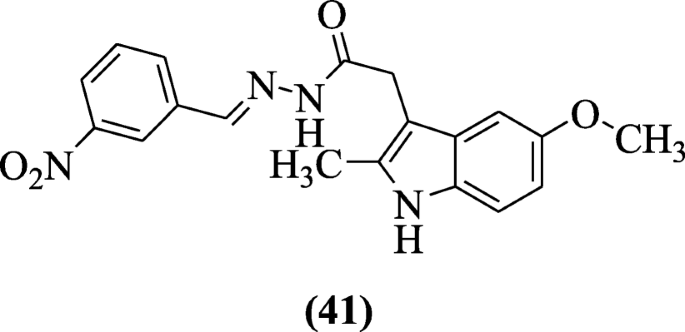
2-(v-Methoxy-2-methyl-1H-indol-iii-yl)-Northward'-[(E)-(substituted phenyl)methylidene] acetohydrazide derivatives were reported by Bhat et al. and investigated for cyclooxygenase expression, lipid peroxidation, ulcerogenic, analgesic, and anti-inflammatory activities. Chemical compound (E)-North'-(3-nitrobenzylidene)-2-(v-methoxy-two-methyl-oneH-indol-3-yl) acetohydrazide (41) was active every bit analgesic and anti-inflammatory agents [24].
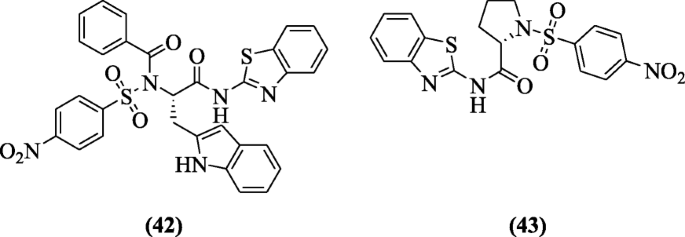
Benzothiazole containing benzene sulphonamide and carboxamide were prepared past Ugwu et al. and evaluated for their in vivo anti-inflammatory, analgesic, and ulcerogenic activities. Amongst the derivatives, compounds (S)-Northward-(1-(benzo[d]thiazol-2-ylamino)-3-(1H-indol-two-yl)-1-oxopropan-2-yl)-N-(4-nitrophenylsulfonyl)benzamide (42) and (S)-North-(benzo[d]thiazol-2-yl)-ane-(4-nitrophenylsulfonyl)pyrrolidine-ii-carboxamide (43) showed anti-inflammatory and analgesic activities along with ulcerogenic index (0.82 and 0.89) compared with indomethacin and celecoxib. In molecular docking studies, interaction is excellent with receptors [25].
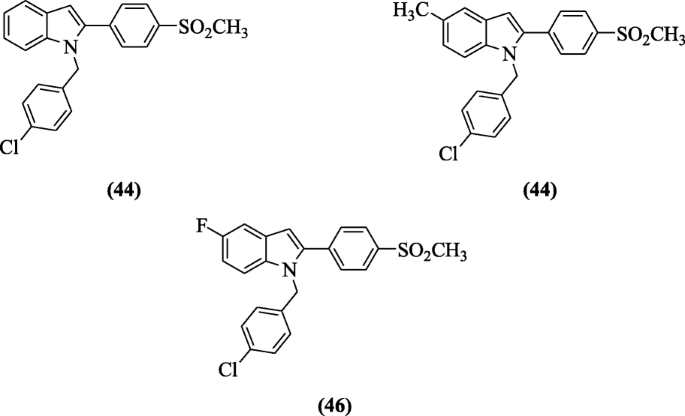
Indomethacin analogs of ii-(4-(methylsulfonyl)phenyl)-1-substituted-indole were synthesized by Shaker et al. and assessed for their in vitro COX-two inhibitory act as well as in vivo anti-inflammatory activity. COX inhibitory activity (in vitro) evaluation showed selective binding with receptor (COX-2) with SI = thirty.35–107.63 as compared to standard drug (SI = 0.079) whereas in vivo anti-inflammatory action studies reported compounds 1-(four-chlorobenzyl)-two-(4-(methylsulfonyl)phenyl)-iH-indole (44) (90.five%), 1-(four-chlorobenzyl)-5-methyl-two-(4-(methylsulfonyl)phenyl)-iH-indole (45) (75.half dozen%), 1-(four-chlorobenzyl)-v-fluoro-ii-(4-(methylsulfonyl)phenyl)-oneH-indole (46), and (81.1%) as most agile. Molecular modeling studies of the compounds 44 and 46 showed excellent bounden interaction to COX-two enzyme [26].
Anticancer activity
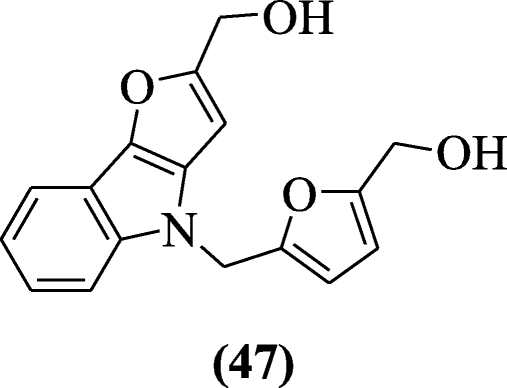
Zhuang et al. reported a series of 2, 4-disubstituted furo[3,2-b]indoles for anticancer action confronting the (man NCI-sixty ) tumor cell lines. Among the tested compounds, compound (5-((two-(hydroxymethyl)-fourH-furo[iii,2-b]indol-4-yl)methyl)furan-ii-yl)methanol (47) demonstrated the best anticancer activity. The analysis of results suggests that the fingerprint of the compound 48 is similar NSC-754549 [27].
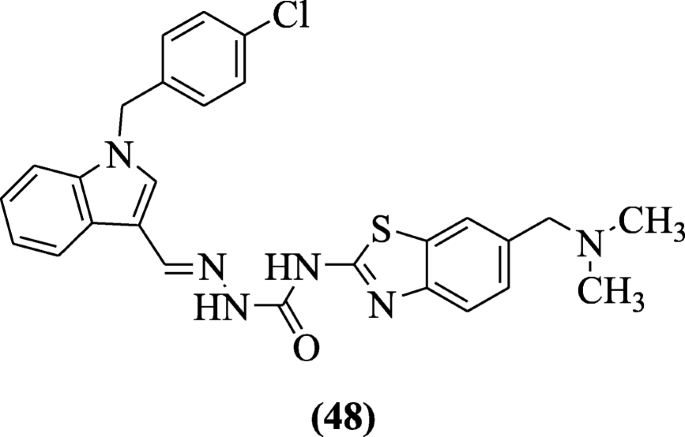
Indole-based derivatives of benzothiazole designed by Ma et al. showed antitumor action against cancer cells breast MDA-MB-231, lung cells (H460), and colon cells (HT29) in MTT assay with positive controls as PAC-i and oncrasin-1. Chemical compound (Eastward)-1-((1-(iv-chlorobenzyl)-iH-indol-3-yl) methylene)-4-(vi-((dimethylamino) methyl) benzo[d]thiazol-two-yl) semicarbazide (48) showed greatest antitumor activeness [28].
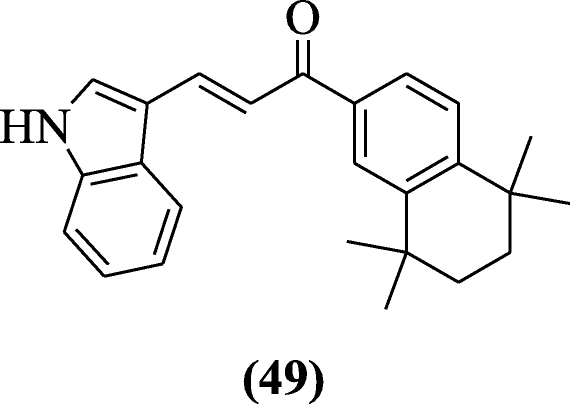
Novel (E)-3-(5-substituted-1H-indol-iii-yl)-i-(5, 5, 8, 8-tetramethyl-five, half-dozen, 7, 8-tetrahydronaphthalen-ii-yl) prop-ii-en-1-one derivatives were prepared and examined for their anticancer effects past Gurkan-Alp et al. Compound (East)-three-(1H-indol-3-yl)-1-(v,v,eight,viii-tetramethyl-5,vi,vii,8-tetrahydronaphthalen-ii-yl)prop-two-en-1-one (49) was found to exist the most active [29].
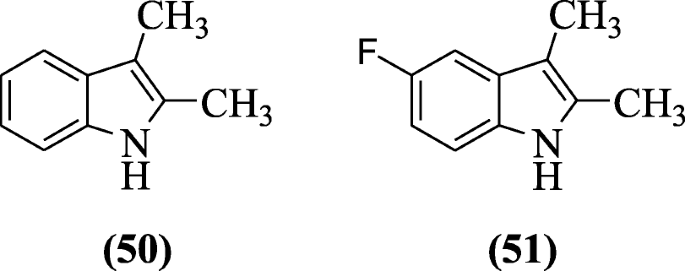
ii,3-Dimethylindoles and tetrahydrocarbazoles as well show anticancer properties against the cancer cell lines such as MCF10A, Calu1, HCT116, Panc1, ACHN, and H460 by using staining assay of propidium iodide (PI) as reported past Kumar et al. Compounds 2,3-dimethyl-1H-indole (50) and 5-fluoro-2,3-dimethyl-1H-indole (51) were plant to exist cytotoxic against cancer cell lines [30].
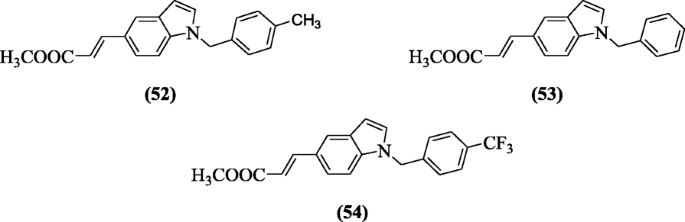
five-(2-Carboxyethenyl) indole derivatives gave anticancer response against HT-29 and K562 jail cell lines as explained by Han et al. and compounds (East)-methyl 3-(one-tolyl-iH-indol-5-yl)acrylate (52), (E)-methyl 3-(1-benzyl-1H-indol-5-yl)acrylate (53) and (Due east)-methyl 3-(i-(4-(trifluoromethyl)benzyl)-1H-indol-five-yl)acrylate (54) demonstrated notable action in cell lines(HT-29) with potency 4.67, 8.24 and 6.73 μM, respectively [31].
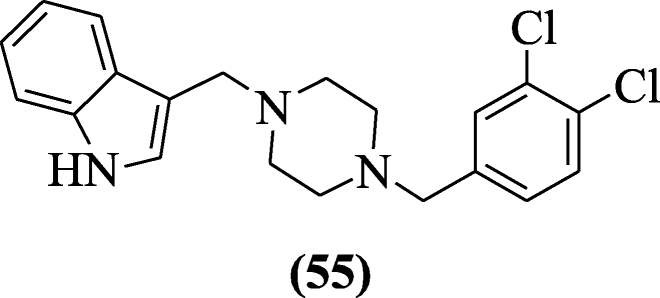
iii-[(iv-Substitutedpiperazin-ane-yl) methyl]-aneH-indole derivatives were prepared using Mannich reaction by Akkoc et al. and evaluated for cytotoxic activity. The cytotoxicity of the compound was dependent on three jail cell lines: human liver (HUH7), chest (MCF7), and colon (HCT116). The almost potent compound against cancer jail cell lines was iii-((4-(iii,four-dichlorobenzyl)piperazin-1-yl)methyl)-iH-indole (55) [32].
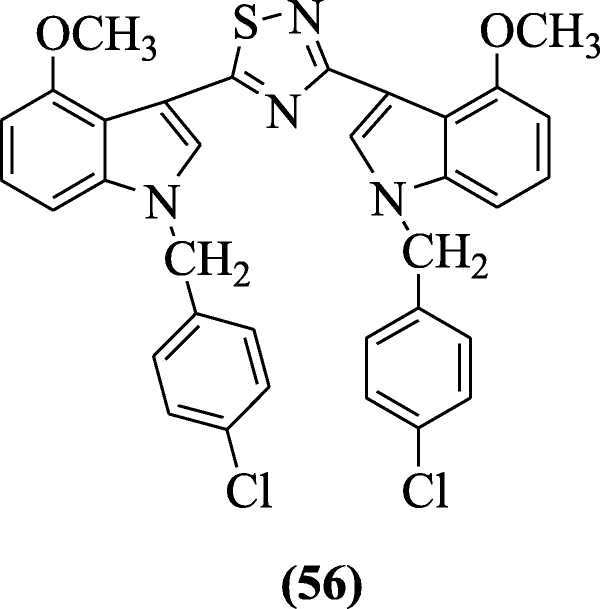
three, v-Bis (indolyl)-one, 2, 4-thiadiazoles showed cytotoxicity against selected human cancer prison cell lines reported by Kumar et al. and compound one-(4-chlorobenzyl)-3-(5-(1-(4-chlorobenzyl)-4-methoxy-aneH-indol-3-yl)-1,2,4-thiadiazol-3-yl)-4-methoxy-aneH-indole (56) gave the most stiff activity (anticancer) [33].
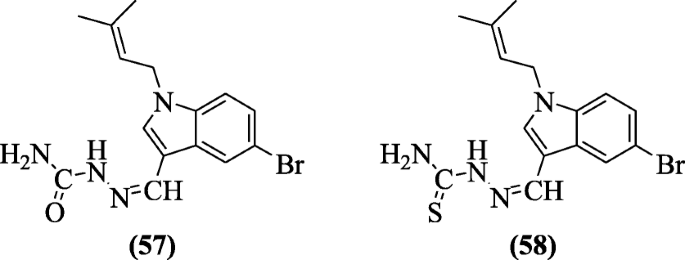
N-1 and C-iii substituted indole derivatives also showed cytotoxic properties as reported by Choppara et al. Compounds (Z)-1-((5-bromo-one-(3-methylbut-2-enyl)-1H-indol-3-yl)methylene)semicarbazide (57) and (Z)-1-((5-bromo-1-(3-methylbut-2-enyl)-1H-indol-iii-yl)methylene)thiosemicarbazide (58) were found to be cytotoxic [34].
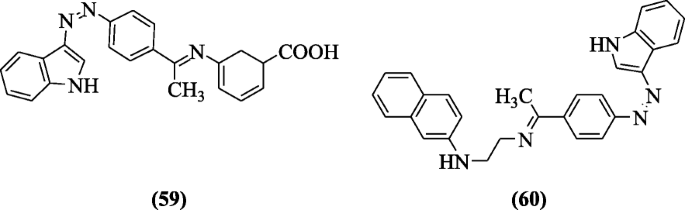
Indole hybridized diazenyl derivatives were designed and reported for cytotoxicity against human cell lines, i.e., leukemic prison cell (K562), normal cell (HEK293), lung jail cell (HCT-116), and breast cell (MDAMB231) adopting MTT assay past Kaur et al. Compounds (R)-5-(((E)-1-(4-((Z)-(1H-indol-three-yl)diazenyl)phenyl)ethylidene)amino)cyclohexa-2,iv-diene-ane-carboxylic acid (59) and Northward-(2-(((E)-1-(iv-((Z)-(oneH-indol-iii-yl)diazenyl)phenyl)ethylidene)amino)ethyl)naphthalen-2-amine (60) showed potential against chest cancer prison cell line (MDAMB231) [35].
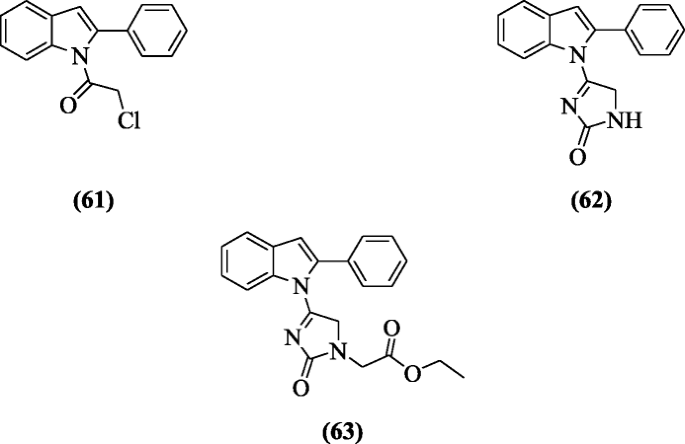
The derivatives of 2-phenylindole containing triazine, thiazolo-due south-triazine, imidazole sugar, imidazolothiazole, and imidazole were prepared and screened for their anticancer activity against colorectal carcinoma, liver carcinoma, prostate cancer, and breast adenocarcinoma by Yousif et al. The compounds 2-chloro-1-(ii-phenyl-1H-indol-1-yl)ethanone (61), 4-(2-phenyl-1H-indol-1-yl)-aneH-imidazol-2(5H)-i (62) and ethyl 2-(2-oxo-4-(two-phenyl-1H-indol-1-yl)-twoH-imidazol-1(5H)-yl)acetate (63) showed high cytotoxic activity [36].
Anti-HIV activity
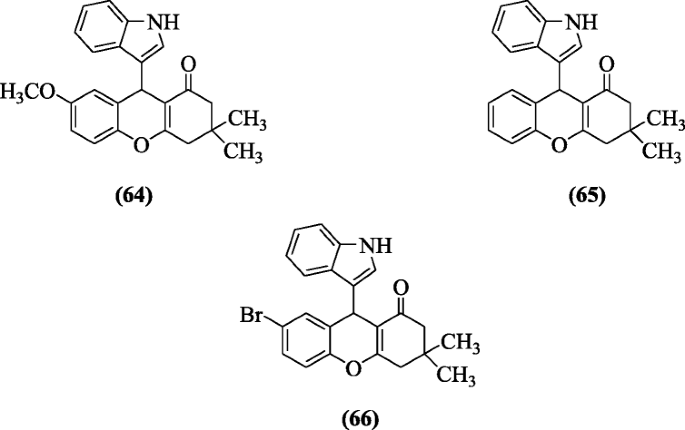
Kasralikar et al. reported a series of novel indolyl and oxochromenyl xanthenone derivatives and performed their molecular docking studies equally an anti-HIV-1. In tested compounds, compounds ix-(1H-indol-3-yl)-vii-methoxy-3,3-dimethyl-3,4-dihydro-2H-xanthen-1(9H)-one (64), 9-(oneH-indol-three-yl)-3,iii-dimethyl-iii,4-dihydro-2H-xanthen-i(9H)-one (65) and vii-bromo-9-(iH-indol-3-yl)-iii,3-dimethyl-3,4-dihydro-2H-xanthen-i(nineH)-i (66) were found to be nigh active compounds [37].
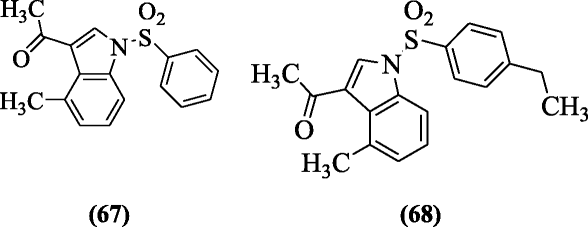
N-arylsulfonyl-3-acetylindole derivative was prepared and evaluated as HIV-1 inhibitors analogs past Ran et al. Compounds one-(4-methyl-ane-(phenylsulfonyl)-1H-indol-3-yl)ethanone (67) and 1-(1-(four-ethylphenylsulfonyl)-iv-methyl-1H-indol-3-yl)ethanone (68) were the most constructive confronting the anti-HIV-1 activity. SAR showed that acetyl group derivatives were more than active [38].
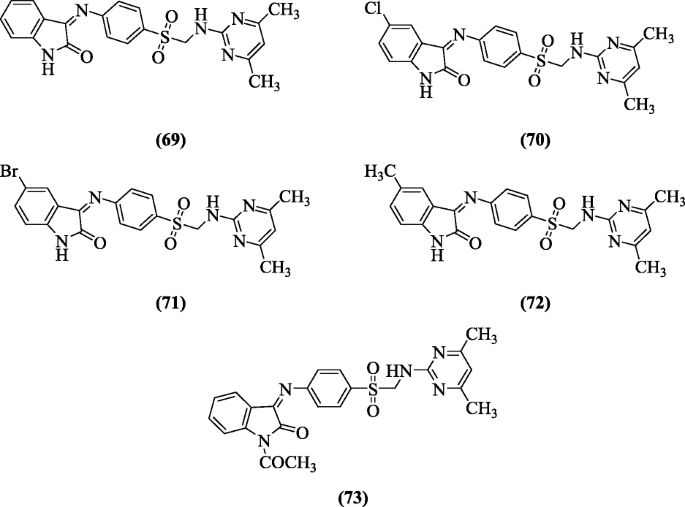
4-[(1,2-Dihydro-2-oxo-3H-indol-3-ylidene)amino]Due north(4,vi-dimethyl-ii-pyrimidinyl)-benzene derivatives were synthesized and screened for their anti-HIV activity confronting HIV-1 (IIIB) and HIV-ii (ROD) strains replication in acutely infected cells (MT-iv) past Selvam et al. Compounds (Z)-3-(iv-((four,6-dimethylpyrimidin-2-ylamino)methylsulfonyl)phenylimino)indolin-two-1 (69), (Z)-5-chloro-3-(4-((4,6-dimethylpyrimidin-2-ylamino)methylsulfonyl)phenylimino)indolin-ii-one (seventy), (Z)-5-bromo-3-(4-((4,6-dimethylpyrimidin-2-ylamino)methylsulfonyl)phenylimino)indolin-2-ane (71), (Z)-3-(4-((4,vi-dimethylpyrimidin-2-ylamino)methylsulfonyl)phenylimino)-5-methylindolin-2-one (72) and (Z)-1-acetyl-3-(iv-((4,6-dimethylpyrimidin-2-ylamino)methylsulfonyl)phenylimino)Indolin-2-one (73) were found to be most effective [39].
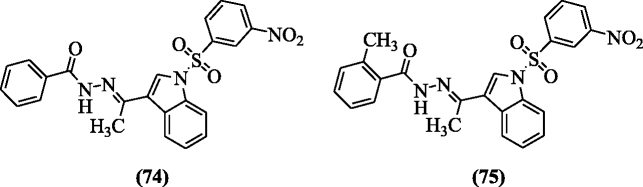
N-Arylsulfonyl-3-acylindole benzoyl hydrazone derivatives were reported equally HIV-1 inhibitors by Che et al. Amongst the reported analogs, compounds (E)-Northward'-(1-(one-(iii-nitrophenylsulfonyl)-1H-indol-3-yl)ethylidene)benzohydrazide (74) and (E)-2-methyl-North'-(1-(1-(3-nitrophenylsulfonyl)-iH-indol-3-yl)ethylidene)benzohydrazide (75) displayed the highest ICfifty and therapeutic index (TI) values 0.26, 769.23, and 0.31, 645.16 in microgram for anti-HIV-ane respectively [xl].
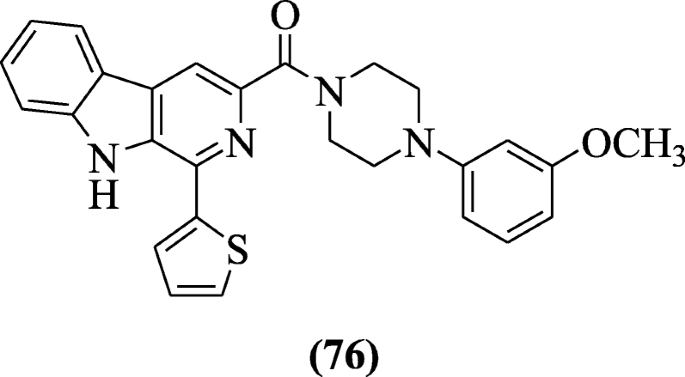
i-(Thiophen-two-yl)-9H-pyrido[three,4-b]indole derivatives were synthesized and screened for their anti-HIV action by Ashok et al. and structure–activity relationship (SAR) studies stated that electron-withdrawing group and electron-donating ortho, para directing groups increases the antiviral activities. Amidst the synthesized derivatives, derivative (4-(3-methoxyphenyl)piperazin-i-yl)(1-(thiophen-two-yl)-nineH-pyrido[3,4-b]indol-three-yl)methanone (76) showed significant anti-HIV activity with selectivity alphabetize (SI) 483 and IC50 (0.53 μM). Lipinski rule is followed by these compounds in the molecular predication studies (In-silico) [41].
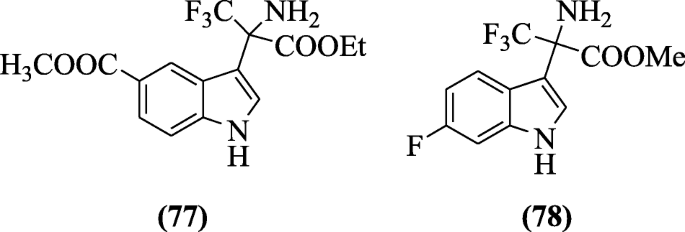
Indole-based contrary transcriptase inhibitors (non-nucleoside) were prepared and tested for anti-HIV virus type HIV-1IIIB using TZM-bl cell assay past Han et al. SAR studies showed that substituent affects the say-so. From the synthesized compounds, compounds methyl two-amino-3, 3, 3-trifluoro-2-(6-fluoro-iH-indol-3-yl) propanoate (77) and methyl three-(2-amino-3-ethoxy-1, i, one-trifluoro-3-oxopropan-2-yl)-1H-indole-5-carboxylate (78) were most agile with IC50 values (0.060 μM and 0.045 μM respectively) [42].
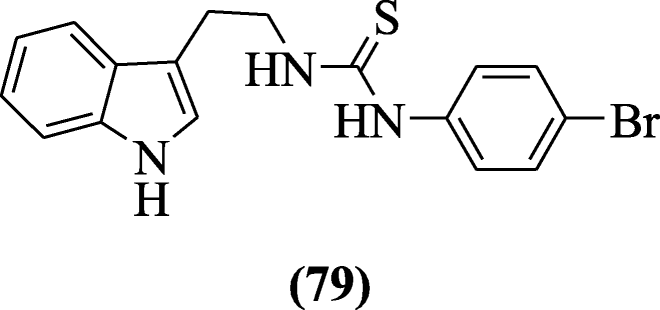
2-(1H-Indol-three-yl) ethylthiourea derivatives were explained as anti-HIV agents by Sanna et al. The compound 1-(ii-(1H-indol-3-yl)ethyl)-3-(4-bromophenyl)thiourea (79) showed strong activity against HIV-1 [43].
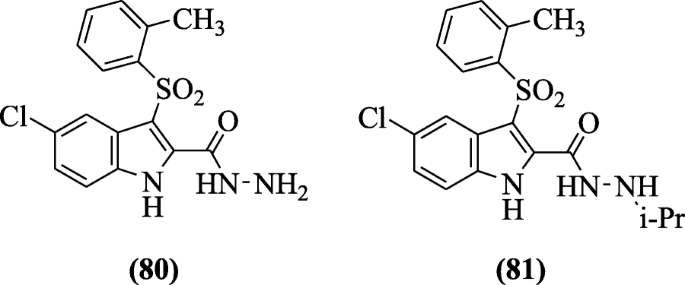
Indolyl aryl sulfones were discussed through molecular modeling studies using 3-D QSAR model as new anti-HIV agents by Ragno et al. From the screened compounds, compounds v-chloro-iii-(o-tolylsulfonyl)-1H-indole-ii-carbohydrazide (80) and 5-chloro-North'-isopropyl-three-(o-tolylsulfonyl)-1H-indole-2-carbohydrazide (81) were found to be the well-nigh potent compound against C-8166 and MT-4 jail cell [44].
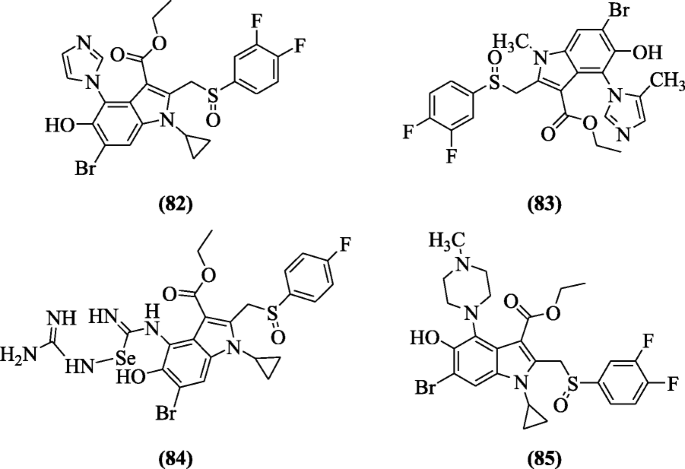
Ethyl six-bromo-5-hydroxy-1H-indole-iii-carboxylates derivatives were prepared and investigated for anti-hepatic activities by Chai et al. Compounds ethyl six-bromo-1-cyclopropyl-2-((3,four-difluorophenylsulfinyl)methyl)-five-hydroxy-4-(1H-imidazol-ane-yl)-1H-indole-3-carboxylate (82), ethyl six-bromo-two-((3,four-difluorophenylsulfinyl)methyl)-five-hydroxy-1-methyl-4-(five-methyl-oneH-imidazol-i-yl)-aneH-indole-3-carboxylate (83), ethyl vi-bromo-1-cyclopropyl-2-(((four-fluorophenyl)sulfinyl)methyl)-4-(((guanidinoselanyl)(imino)methyl)amino)-five-hydroxy-1H-indole-3-carboxylate (84), and ethyl vi-bromo-ane-cyclopropyl-2-((iii,4-difluorophenylsulfinyl)methyl)-five-hydroxy-4-(iv-methylpiperazin-1-yl)-iH-indole-3-carboxylate (85) possessed meaning activities with IC50 (iii.six, half dozen.37, five.ii, and 5.four μg/ml) confronting hepatitis B virus (HBV) [45].
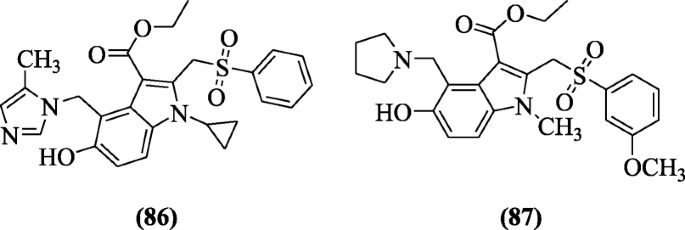
Ethyl v-hydroxy-1H-indole-iii-carboxylates derivatives were as well explained for their hepatitis inhibitory activities in cells by Zhao et al. The structures ethyl 1-cyclopropyl-5-hydroxy-4-((five-methyl-1H-imidazol-i-yl)methyl)-two-(phenylsulfonylmethyl)-1H-indole-3-carboxylate (86) and ethyl five-hydroxy-2-((three-methoxyphenylsulfonyl)methyl)-1-methyl-4-(pyrrolidin-1-ylmethyl)-1H-indole-3-carboxylate (87) exhibited meaning activity against hepatitis B virus (HBV) with the IC50 values of compounds (24.xc μg/ml) and (fifteen.41 μg/ml) higher than those of the used of reference drug lamivudine (228.00 μg/ml) [46].
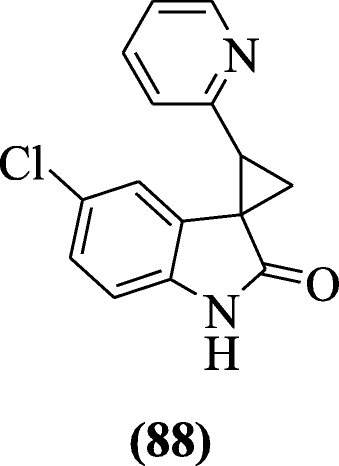
Heterocycle-containing oxindoles derivatives were prepared and evaluated for their anti-HIV action by Jiang et al. In this series, compound (1Southward, 2S)-five'-chloro-2-(pyridin-ii-yl)spiro[cyclopropane-one,3'-indolin]-2'-one (88) exhibited stiff inhibitory activities against viruses [47].
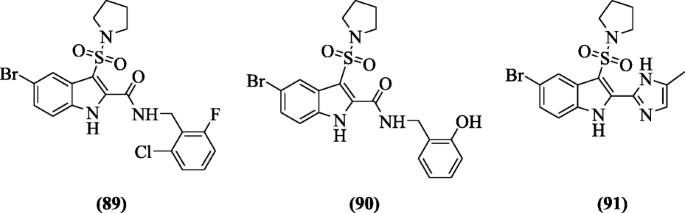
Indole sulfonamides derivatives were designed and evaluated as non-nucleoside reverse transcriptase inhibitors (NNRTIs) by Zhao et al. Amid all, the analogs North-(ii-chloro-6-fluorobenzyl)-5-bromo-3-(pyrrolidin-1-ylsulfonyl)-aneH-indole-2-carboxamide (89), Northward-(2-hydroxybenzyl)-5-bromo-3-(pyrrolidin-ane-ylsulfonyl)-iH-indole-2-carboxamide (ninety), and v-bromo-2-(five-methyl-iH-imidazol-2-yl)-iii-(pyrrolidin-1-ylsulfonyl)-1H-indole (91) improved the activities against HIVRT mutants Y181C and K103N which retain potent cellular activeness [48].
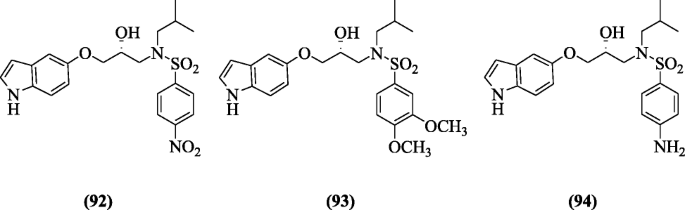
(R)-N-(3-(oneH-indol-5-yloxy)-two-hydroxypropyl)-N-isobutyl benzenesulfonamide derivatives were screened for anti-HIV action past Chiummiento et al. Among all compounds in this series, compounds (R)-N-(3-(iH-indol-5-yloxy)-ii-hydroxypropyl)-Due north-isobutyl-4nitrobenzenesulfonamide (92), (R)-N-(iii-(oneH-indol-5-yloxy)-2-hydroxypropyl)-Due north-isobutyl-3,4-dimethoxybenzenesulfonamide (93), and (R)-Northward-(3-(aneH-indol-5-yloxy)-2-hydroxypropyl)-4-amino-Northward-isobutylbenzenesulfonamide (94) demonstrated the all-time results [49].
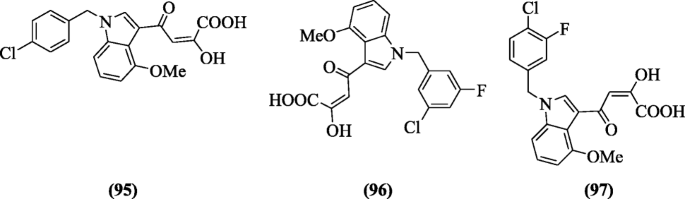
(Eastward)-4-(one-(Substitutedbenzyl)-4-methoxy-1H-indol-three-yl)-ii-hydroxy-four-oxobut-2-enoic acid derivatives were reported as new HIV inhibitor (integrase strand-transfer inhibitors) past Ferro et al. The binding modes of the compounds were studied by induced-fit docking (IFD). Among all compounds, (East)-4-(1-(4-chlorobenzyl)-4-methoxy-1H-indol-3-yl)-2-hydroxy-4-oxobut-two-enoic acid (95), (East)-4-(1-(iii-chloro-5-fluorobenzyl)-4-methoxy-1H-indol-iii-yl)-2-hydroxy-4-oxobut-ii-enoic acrid (96), and (E)-4-(ane-(4-chloro-3-fluorobenzyl)-4-methoxy-aneH-indol-iii-yl)-two-hydroxy-four-oxobut-2-enoic acid (97) showed inhibition (in strand-transfer) in respect to elvitegravir [50].
Antioxidant activity
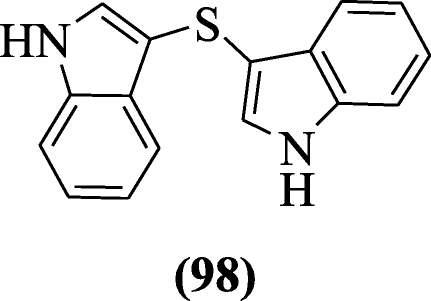
Di(iH-indol-3-yl)sulfane derivatives were prepared and evaluated as antioxidants agents by Silveira et al. Compound di(1H-indol-3-yl)sulfane (98) exhibited antioxidant activity in ferric reducing ability of plasma (FRAP), 2,ii-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-Azino-bis(three-ethylbenzothiazoline-6-sulfonic acrid ) (ABTS) assays at micromolar concentration [51].
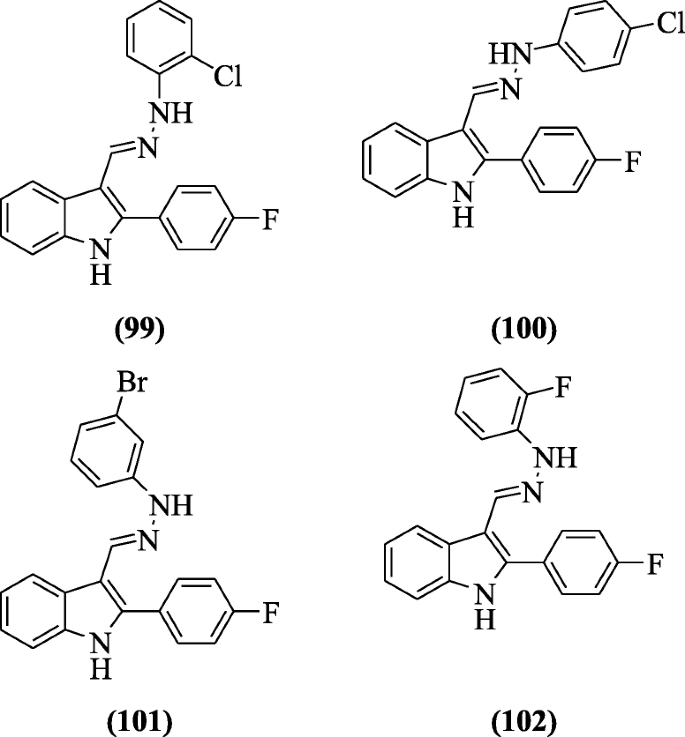
(E)-1-(Substitutedphenyl)-ii-((ii-(4-fluorophenyl)-iH-indol-3-yl)methylene)hydrazine derivatives equally MLT (Melatonin) analogs were prepared by Gurer-Orhan et al. and were tested for antioxidant activity in homo erythrocytes. Compounds (East)-1-(two-chlorophenyl)-2-((ii-(4-fluorophenyl)-oneH-indol-three-yl)methylene)hydrazine (99), (E)-1-(4-chlorophenyl)-2-((2-(iv-fluorophenyl)-aneH-indol-3-yl)methylene)hydrazine (100), (E)-1-(3-bromophenyl)-two-((ii-(4-fluorophenyl)-1H-indol-3-yl)methylene)hydrazine (101), and (Due east)-1-(ii-fluorophenyl)-2-((two-(four-fluorophenyl)-1H-indol-3-yl)methylene)hydrazine (102) exhibited pregnant action [52].
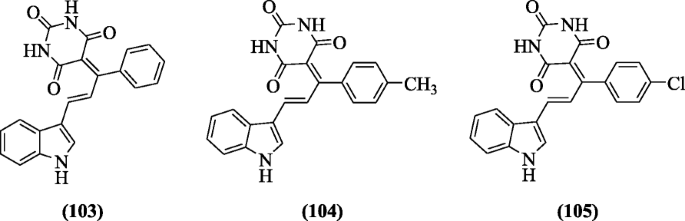
(E)-5-(one-(Substitutedphenyl)-3-(1H-indol-3-yl) allylidene) pyrimidine-2, 4, 6(oneH, 3H, fiveH)-trione derivatives containing barbitone moiety were reported equally Deoxyribonucleic acid cleavage and antioxidant agents past Biradar et al. The compounds (E)-v-(three-(1H-indol-3-yl)-1-phenylallylidene)pyrimidine-2,4,six(iH,3H,5H)-trione (103), (East)-five-(3-(iH-indol-3-yl)-1-p-tolylallylidene)pyrimidine-ii,four,half-dozen(1H,iiiH,5H)-trione (104) and (E)-5-(1-(iv-chlorophenyl)-iii-(1H-indol-three-yl)allylidene)pyrimidine-2,four,6(oneH,3H,5H)-trione (105) exhibited splendid antioxidant and Dna cleavage activities [53].
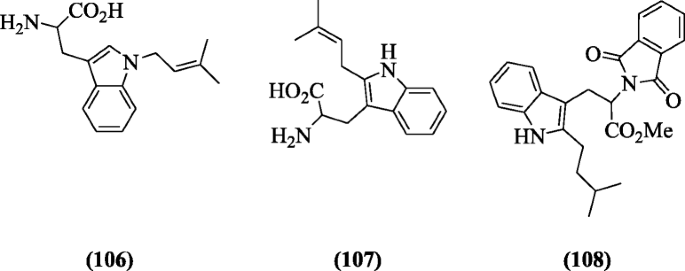
Indole derivatives like tryptophan and tryptamine were investigated for Dna clevaging activity by Estevão et al. Compounds ii-amino-iii-(1-(3-methylbut-ii-enyl)-1H-indol-3-yl)propanoic acid (106) (IC504.13 ± 0.17 μM), ii-amino-3-(2-(3-methylbut-2-enyl)-aneH-indol-3-yl)propanoic acid (107) (ICl 4.56 ± 0.48 μM), and methyl 2-(ane,iii-dioxoisoindolin-ii-yl)-three-(two-isopentyl-oneH-indol-iii-yl)propanoate (108) (IC50xiv.0 ± 6.viii μM ) showed meaning action [54].
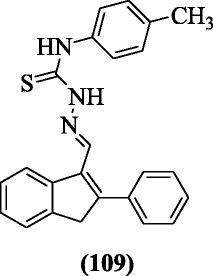
(E)-1-((ii-Phenyl-3H-inden-1-yl) methylene)-iv-substitutedthiosemicarbazides, a new class of antioxidant agents, were synthesized by Bakherad et al. and compounds exhibited better anti-oxidant activities. Compound (E)-1-((2-phenyl-3H-inden-1-yl)methylene)-iv-p-tolylthiosemicarbazide (109) found to be the well-nigh potent compound [55].
(E)-one-(Substitutedphenyl)-2-((1-methyl-1H-indol-ii-yl)methylene)hydrazines were synthesized and reported as antioxidant agents by Suzen et al., and compounds (E)-1-(ii,5-difluorophenyl)-2-((1-methyl-1H-indol-ii-yl)methylene)hydrazine (110), (East)-1-(2,v-dichlorophenyl)-2-((1-methyl-1H-indol-2-yl)methylene)hydrazine (111) and (East)-1-(2,6-dichlorophenyl)-2-((1-methyl-1H-indol-2-yl)methylene)hydrazine (112) were the most promising compounds [56].
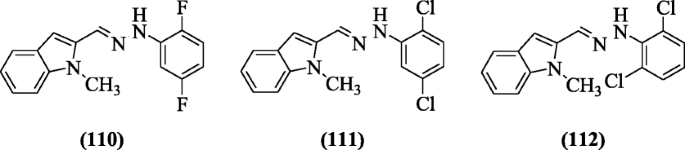
5-Chloroindole hydrazide/hydrazone derivatives were prepared and evaluated for antioxidant action past Yılmaz et al. 2,two-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activeness (IC50 two to sixty μM) was shown by all compounds. Chemical compound (E)-one-((v-chloro-1H-indol-two-yl) methylene)-2-(two-chlorophenyl) hydrazine (113) possessed high inhibitory activities in assay (LP) against melatonin at 0.i mM [57].
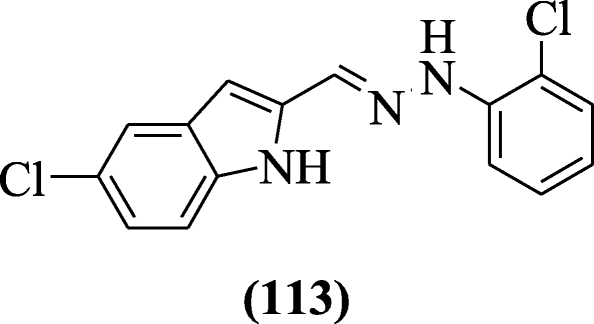
three-(i-(4-(4-chlorophenyl)thiazol-2-yl)-three-(substitutedphenyl)-1H-pyrazol-five-yl)-aneH-indole derivatives were reported as antioxidant agents by Ummadi et al. Compounds 3-(1-(4-(four-chlorophenyl)thiazol-2-yl)-3-p-tolyl-oneH-pyrazol-five-yl)-1H-indole (114) and 3-(1-(4-(4-chlorophenyl)thiazol-2-yl)-3-(4-methoxyphenyl)-aneH-pyrazol-5-yl)-oneH-indole (115) showed excellent antioxidant activity in comparison to ascorbic acid. Structural activity human relationship (SAR) stated that OCHiii, CH3 groups showed college action (scavenging) as compared to NO2, Cl, Br groups [58].
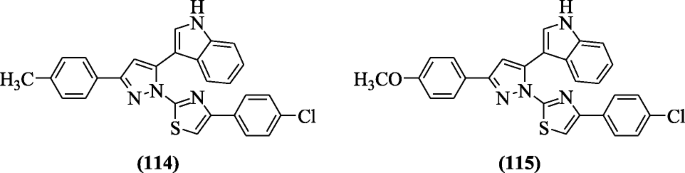
Melatonin retinamide derivatives were synthesized through reaction between the melatonin and tetrahydrotetramethylnaphthalene carboxylic acid and screened for antioxidant activity by Ates-Alagoz et al. The compounds have weak 2,two-Diphenyl-1-picrylhydrazyl (DPPH) inhibition action design and some of the compounds N-(ii-(1H-indol-3-yl)ethyl)-five,v,viii,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-ii-carboxamide (116), N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-v,5,8,8-tetramethyl-five,vi,7,viii-tetrahydronaphthalene-2-carboxamide (117), Northward-(1H-indol-5-yl)-five,5,8,8-tetramethyl-5,half-dozen,7,8-tetrahydronaphthalene-2-carboxamide (118), Due north-(3,5,5,viii,8-pentamethyl-5,6,7,8-tetrahydronaphthalen-ii-yl)-1H-indole-2-carboxamide (119), 4-(aneH-indol-3-yl)-Northward-(three,v,five,viii,8-pentamethyl-5,6,7,viii-tetrahydronaphthalen-2-yl)butanamide (120), and 5-(1H-indol-3-yl)-N-(3,5,5,8,eight-pentamethyl-5,vi,vii,8-tetrahydronaphthalen-two-yl)pentanamide (121) possessed a strong inhibition of lipid peroxidation (88, 96, xc, 94, 93, and 86%) [59].
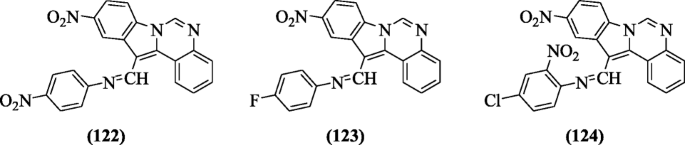
N-((10-nitro-1H-indolo [i, 2-c]quinazolin-12-yl)methylene)benzenamines were prepared and tested for their anti-oxidant activity by Dixit et al. Nearly all derivatives have shown good antioxidant activity at all the concentrations, but compounds 4-nitro-N-((x-nitroH-indolo[ane,two-c]quinazolin-12-yl)methylene)benzenamine (122), 4-fluoro-Northward-((ten-nitroH-indolo[one,two-c]quinazolin-12-yl)methylene)benzenamine (123), and four-chloro-2-nitro-N-((10-nitroH-indolo[one,2-c]quinazolin-12-yl)methylene)benzenamine (124) were found to exist the all-time complimentary radical scavengers [threescore].
Antimicrobial activity
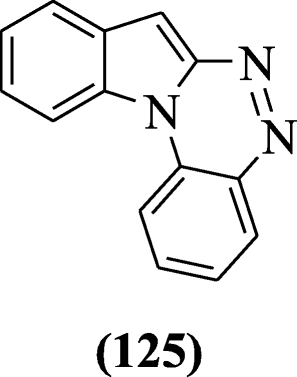
Indole[1,2-c]-1,2,4- butylnitrite benzotriazine derivatives were prepared using Sandmeyer reaction and screened for the antifungal activity by Xu et al. Compound indole[1,2-c]-i,two,four- butylnitrite benzotriazine (125) was more than strong derivative [61].
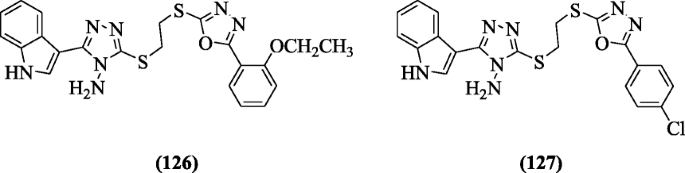
three-(2-(5-(Substitutedphenyl)-ane, iii, 4-oxadiazol-2-ylthio) ethylthio)-5-(1H-indol-3-yl)-4H-1, 2, 4-triazol-4-amines were prepared by Shi et al. using special technique, i.eastward., ultrasonic. Compounds iii-(ii-(five-(2-ethoxyphenyl)-i,iii,four-oxadiazol-two-ylthio)ethylthio)-5-(1H-indol-3-yl)-fourH-1,ii,4-triazol-4-amine (126), and three-(2-(5-(iv-chlorophenyl)-1,3,4-oxadiazol-2-ylthio)ethylthio)-5-(1H-indol-iii-yl)-4H-1,2,4-triazol-4-amine (127) exhibited excellent action confronting Staphylococcus aureus and Escherichia coli strains [62].
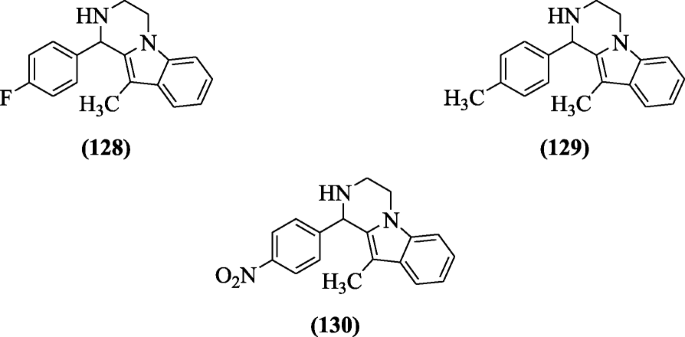
Substituted 1, two, 3, four-tetrahydropyrazino [1, ii-a] indole derivatives were reported past Tiwari et al. as antimicrobial agents against bacteria (both Gram positive and negative). Compounds 1-(four-fluorophenyl)-10-methyl-i,ii,3,four-tetrahydropyrazino[1,2-a]indole (128), 10-methyl-1-p-tolyl-1,2,iii,iv-tetrahydropyrazino[1,2-a]indole (129), and 10-methyl-1-(four-nitrophenyl)-1,2,3,4-tetrahydropyrazino[ane,2-a]indole (130) exhibited significant activity [63].
Methyl (Due east)-1-(substitutedbenzyl)-3-((two-(four, v-dihydro-1H-imidazol-2-yl)hydrazono)methyl)-1H-indole-5-carboxylate derivatives were reported as antimicrobial agents past Hong et al. Compounds (131), (132), (133), and (134) showed activity confronting Mycobacterium tuberculosis, multidrug-resistant Acinetobacter baumanii, and Gram-negative bacteria [64].
Azo dye of indoles were prepared by Ozturk et al and evaluated in vitro against yeast Saccharomyces cerevisiae, Gram (+), and (-) bacteria. Compounds (East)-ethyl 4-((oneH-indol-iii-yl) diazenyl) benzoate (135), (E)-ethyl 4-((1-methyl-iH-indol-3-yl) diazenyl) benzoate (136) and (Z)-1-(iv-methoxyphenyl)-2-(iii-methyl-aneH-indol-ii-yl)diazene (137) showed adept activity [65].
2, 3-Diarylindoles derivatives containing amine substituent at the 5 and 6 positions of indole were prepared and screened equally anticoccidial agents by Scribner et al. Compound 2-(4-fluorophenyl)-6-(piperidin-4-yl)-three-(pyridin-iii-yl)-1H-indole (138) showed the best activity [66].
A series of nitrogen and carbon substituted bisindoles were synthesized and investigated for antimicrobial agents by Singh et al. The Due north-benzyl moiety or morpholine or pyrrolidine at position 3 and xylidine or butane or propane every bit the bridge between the indoles were proficient for activeness. Dockings studies showed strong interactions in the agile sites of topoisomerase Two lanosterol demethylase and dihydrofolate reductase. Compounds (139) and (140) were more than active [67].
Antitubercular activity
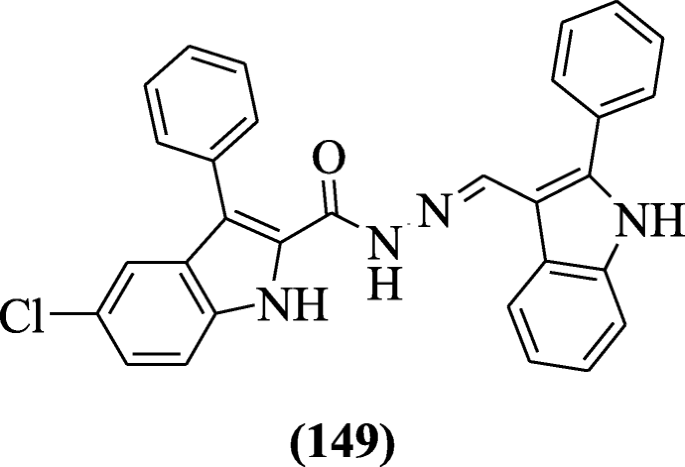
(Due east)-1-(2-(1H-indol-3-yl)-5-(pyridin-4-yl)-1,3,four-oxadiazol-three(2H)-yl)-3-(substitutedphenyl)prop-ii-en-1-one derivatives derived from pyridine and Indole were prepared and investigated in active and dormant state against H37Ra MTB (Mycobacterium tuberculosis) and BCG (Mycobacterium bovis) for their in vitro antitubercular activity by Desai et al. Compounds (Eastward)-1-(ii-(1H-indol-iii-yl)-v-(pyridin-4-yl)-1,3,4-oxadiazol-3(2H)-yl)-3-(2-hydroxyphenyl)prop-two-en-1-one (141), (E)-i-(2-(oneH-indol-3-yl)-5-(pyridin-4-yl)-1,3,four-oxadiazol-three(twoH)-yl)-3-(2-nitrophenyl)prop-two-en-i-one (142), (E)-1-(2-(aneH-indol-3-yl)-5-(pyridin-4-yl)-1,3,4-oxadiazol-3(iiH)-yl)-iii-(four-nitrophenyl)prop-ii-en-1-ane (143), and (E)-one-(2-(1H-indol-3-yl)-5-(pyridin-iv-yl)-ane,3,four-oxadiazol-3(2H)-yl)-3-(ii,4-dichlorophenyl)prop-ii-en-1-one (144) exhibited effective antitubercular activity [68].
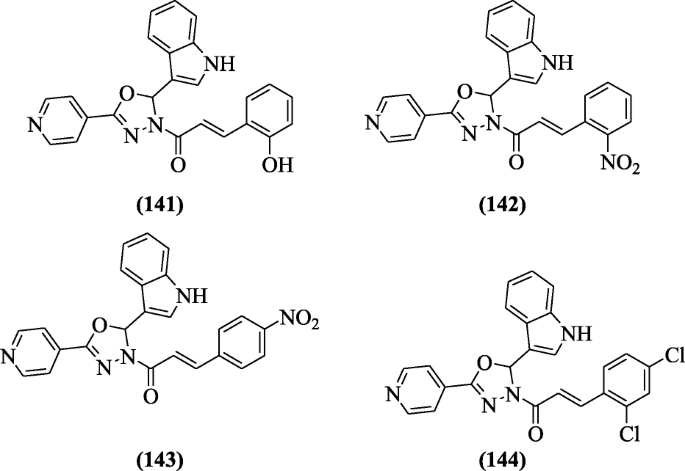
6-Cyano-5-methoxyindolo [2, 3-a] carbazole derivatives were designed and screened for their moderate inhibitory activities confronting H37Rv (Mycobacterium tuberculosis) strain and Bacillus anthracis (ANR) strain by Guo et al. to gainsay tuberculosis and anthrax infections. Compounds half dozen-methoxy-11,12-dihydroindolo[2,3-a]carbazole-5-carbonitrile (145), xi,12-dihydroindolo[2,3-a]carbazole-5-carbonitrile (146), and 11-benzyl-xi,12-dihydroindolo[2,three-a]carbazole-5-carbonitrile (147) displayed moderate activity against Mycobacterium tuberculosis and good activity confronting Bacillus anthracis [69].
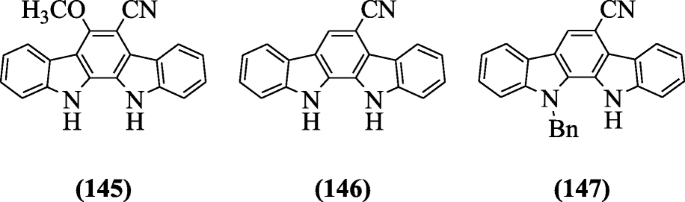
Indole and pyridine nuclei combined through hydrazones-hydrazide and hydrazides were evaluated their in vitro antimycobacterial activity by Velezheva et al. In these series, the most potent compound was ethyl (E)-iii-((two-isonicotinoylhydrazono)methyl)-5-methyl-oneH-indole-two-carboxylate (148) (MIC value of 0.05 μg/mL and selectivity alphabetize 300) was active against Mycobacterium tuberculosis H37Rv [70].
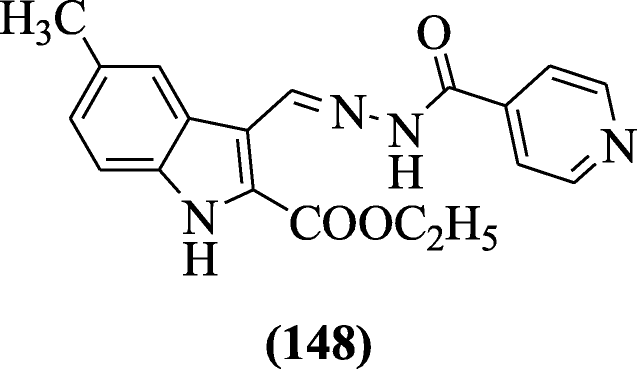
Walmik et al. synthesized several novel N'-((2-phenyl-1H-indol-3-yl) methylene), substituted phenyl-iH-indole-two-carbohydrazide derivatives, and screened for their in vitro antimycobacterial activeness. The antitubercular result showed that chlorine derivatives were most active. Compound five-chloro-3-phenyl-N'-((2-phenyl-1H-indol-3-yl)methylene)-1H-indole-2-carbohydrazide (149) (MIC = 0.2 μg/mL) possessed potent growth inhibitory result confronting H37Rv Mycobacterium tuberculosis [71].
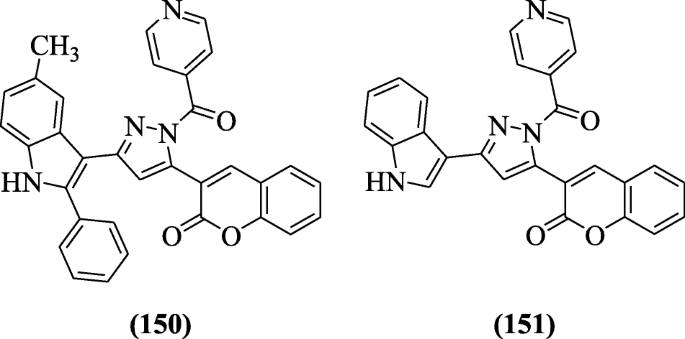
3-(1-Isonicotinoyl-3-(subtituted-ii-phenyl-1H-indol-3-yl)-1H-pyrazol-5-yl)-2H-chromen-2-ane derivatives were synthesized and reported every bit antimycobacterial agents past Rathod et al. In molecular docking studies, the mode of action of these agile derivatives were studied and compounds iii-(1-isonicotinoyl-3-(v-methyl-two-phenyl-1H-indol-three-yl)-1H-pyrazol-5-yl)-2H-chromen-ii-one (150) and three-(3-(aneH-indol-3-yl)-1-isonicotinoyl-iH-pyrazol-v-yl)-twoH-chromen-2-one (151) gave the promising outcome with Mycobacterium tuberculosis H37Rv strain at 12.5 and 25 μg/ml respectively [72].
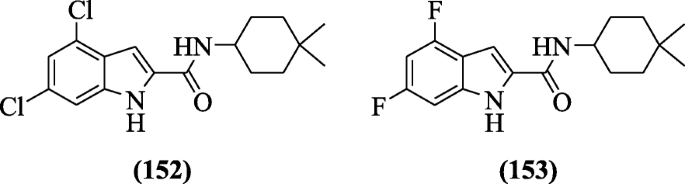
N-(4, 4-dimethylcyclohexyl)-substitutedindole-ii-carboxamides were reported equally antituberculosis agents by Kondreddi et al. Structure-activeness relationship ( SAR) studies revealed that alkyl groups reduced solubility and increased Mycobacterium tuberculosis activity. Compounds 4,6-dichloro-N-(iv,iv-dimethylcyclohexyl)-1H-indole-2-carboxamide (152) and N-(4,four-dimethylcyclohexyl)-4,6-difluoro-1H-indole-two-carboxamide (153) agile compounds displayed improved in vitro activity [73].
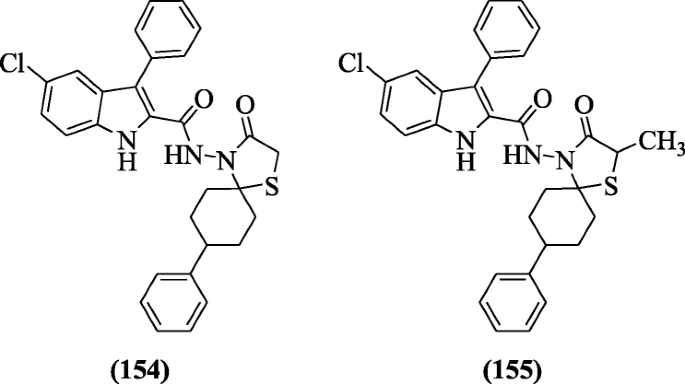
Spirothiazolidinone derivatives of 5-chloro-3-phenyl-1H-indoles were synthesized by Cihan-Üstündağ et al. and evaluated for their in vitro antitubercular action. Among all, compounds 5-chloro-three-phenyl-Northward-(8-phenyl-3-oxo-1-thia-azaspiro[4.5]-decan-iv-yl)-3-phenyl-1H-indole-2-carboxamide (154) (MIC = 3.9 μM) and 5-chloro-Due north-(2-methyl-8-phenyl-iii-oxo-1-thia-4-azaspiro[4.5]-decan-iv-yl)-iii-phenyl-1H-indole-ii-carboxamide (155) (MIC = 7.viii μM) were the almost active compounds having eight-phenyl spiro ring against H37Rv ATCC 27294 (Mycobacterium tuberculosis) [12].
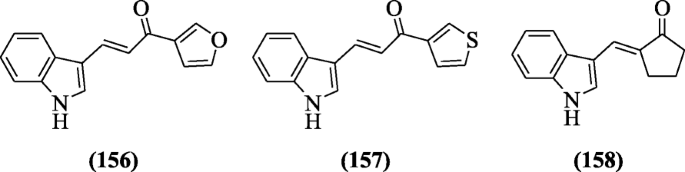
A library of indole chalcones was made by Ramesh et al. and screened for their antimycobacterial activeness opposite H37Rv strain. Compounds (E)-1-(furan-iii-yl)-3-(1H-indol-three-yl)prop-2-en-one-i (156) (MIC = 210 μM),(Due east)-three-(aneH-indol-3-yl)-1-(thiophen-2-yl)prop-ii-en-1-one(157) MIC = 197 μM) and (E)-2-((iH-indol-2-yl)methylene)cyclopentan-i-one (158) (MIC = 236 μM) were establish to be strong drug against Mycobacterium tuberculosis [74].
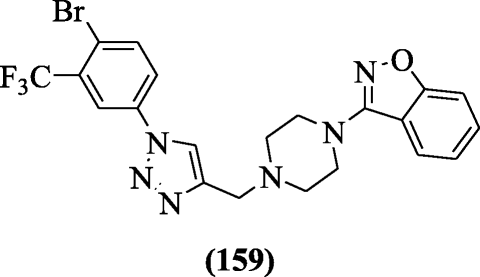
Naidu et al. synthesized various iii-(iv-((one-(4-bromo-3substitutedphenyl)-1H-1, two, 3-triazol-four-yl) methyl) piperazin-one-yl) benzo[d]isoxazole derivatives every bit antitubercular agents against H37Rv strain Mycobacterium tuberculosis. Among the tested compound, chemical compound 3-(4-((1-(4-bromo-iii-(trifluoromethyl)phenyl)-1H-1,ii,iii-triazol-4-yl)methyl)piperazin-1-yl)benzo[d]isoxazole (159) (MIC = 6.16 μM) exhibited best antitubercular activity. Receptor interactions were studied by docking to pantothenate synthetase enzyme [75].
Anticholinesterase activity
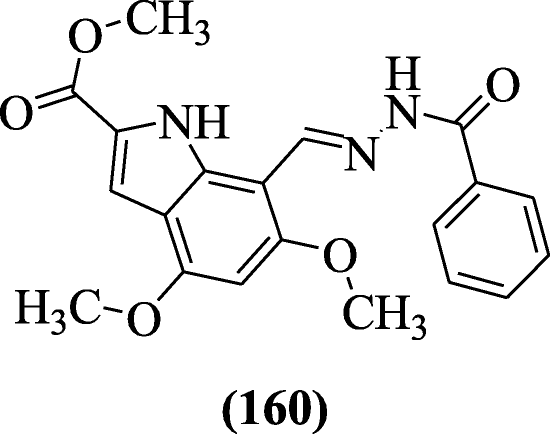
Bingul et al. synthesized a novel iv, half-dozen-dimethoxyindole-based hydrazide hydrazones and evaluated their anticholinesterase activity towards cholinesterase (AChE and BuChE) enzymes. Compound methyl (East)-seven-((2-benzoylhydrazono)methyl)-4,6-dimethoxy-iH-indole-two-carboxylate (160) was nearly active [76].
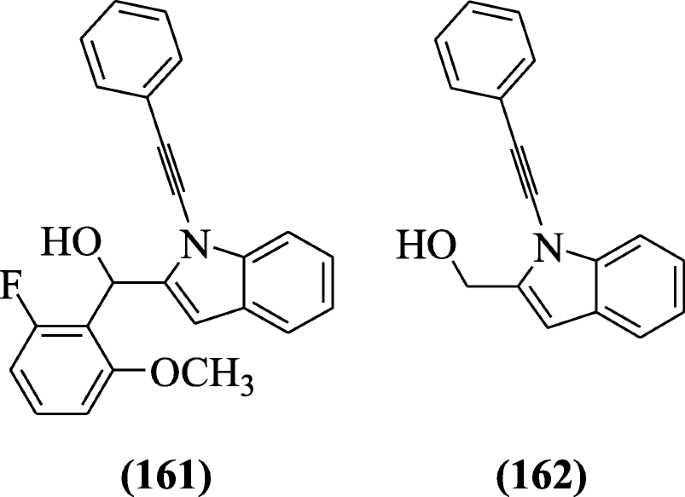
Prochnow et al. prepared 2-substituted-Due north-alkynylindoles and screened for anticholinesterase activity. Derivatives (2-fluoro-half dozen-methoxyphenyl)(1-(2-phenylethynyl)-1H-indol-2-yl)methanol (161) and (1-(2-phenylethynyl)-1H-indol-2-yl)methanol (162) were found as potential inhibitors of cholinesterase activity [77].
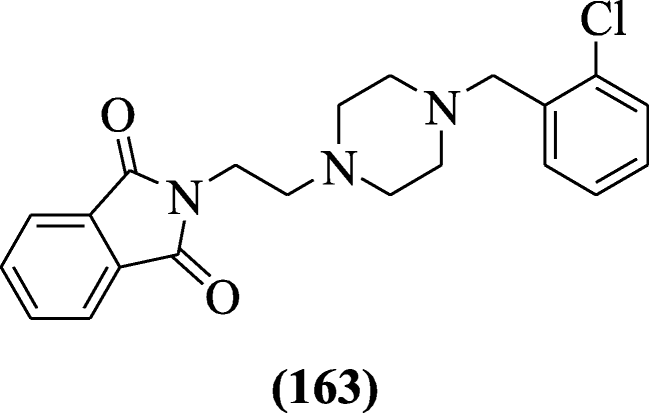
2-(ii-(four-(Substitutedbenzyl) piperazin-1-yl) ethyl) isoindoline-i, 3-dione derivatives were reported as anticholinesterase agents by Mohammadi-Faran et al. Among synthesized derivatives, 2-(two-(4-(ii-chlorobenzyl) piperazin-1-yl) ethyl) isoindoline-one, three-dione (163) (IC50 = 0.91 ± 0.045 μM) exhibited the highest activity with respect to the standard drug (IC50 = 0.fourteen ± 0.03 μM) [78].
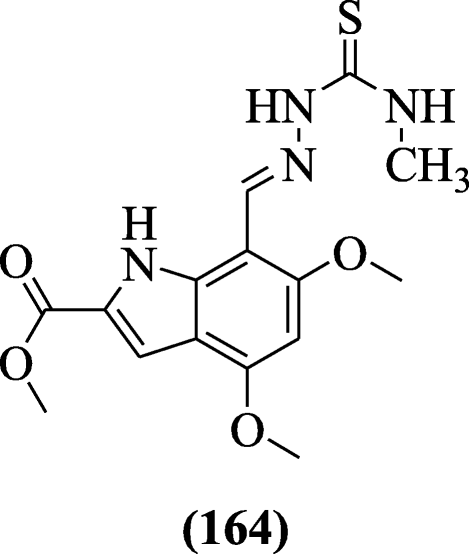
A novel 4, 6-dimethoxyindole-7-thiosemicarbazone derivatives were designed through Schiff base condensation reaction of indole carbaldehydes and thiosemicarbazides and evaluated for anticholinesterase properties by Bingül et al. Compound methyl (E)-four,6-dimethoxy-seven-((2-(methylcarbamothioyl)hydrazono)methyl)-1H-indole-2-carboxylate (164) exhibited moderate inhibition towards acetylcholinesterase and butyrylcholinesterase enzyme [79].
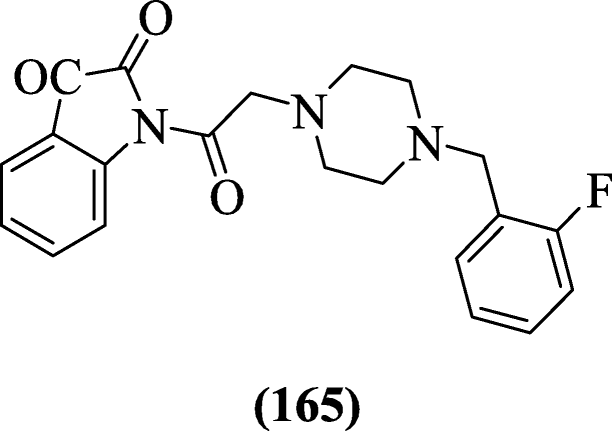
1-(2-(4-(Substitutedbenzyl)piperazin-one-yl)acetyl)indoline-two,3-dione derivatives using piperazine and fluorobenzyl substituents were reported as acetylcholinesterase inhibitor by Ismail et al. Compound 1-(two-(4-(2-fluorobenzyl)piperazin-i-yl)acetyl)indoline-2,three-dione (165) was found to exhibit the most agile compound [80].
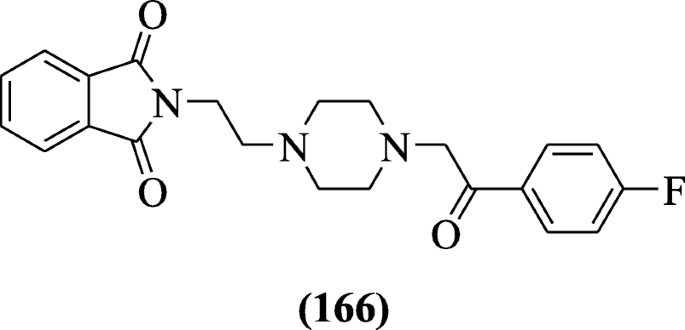
Isoindoline-1, iii-dione derivatives were prepared as an inhibitor of acetylcholinesterase using Ellman'south test by Aliabadi et al. Compound 2-(ii-(4-(2-(4-Fluorophenyl)-2-oxoethyl) piperazin-ane-yl) ethyl) isoindoline-i, 3-dione (166) (ICfifty = sixteen.42 ± 1.07 μM) was active [81].
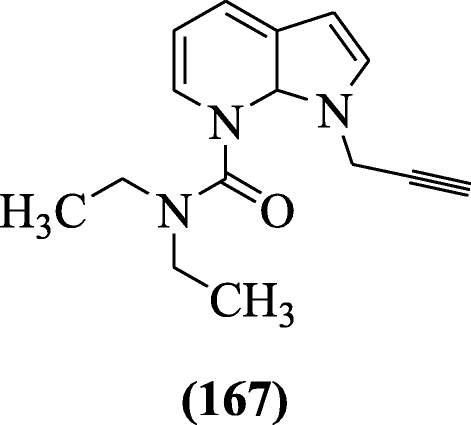
Various urea and carbamates derived indole derivatives were reported for the inhibition of man monoamine oxidase-A (hMAO-A), human monoamine oxidase-B (hMAO-B), acetylcholinesterase (AChE), and butyrylcholinesterase (BuChE) by Denya et al. Molecular modeling showed significant interactions on active site of the enzyme. Compound North, N-Diethyl-Due north′-[1-(prop-2-yn-i-yl)-oneH-indol-vi-yl] urea (167) was most potent [82].
Antimalarial activity
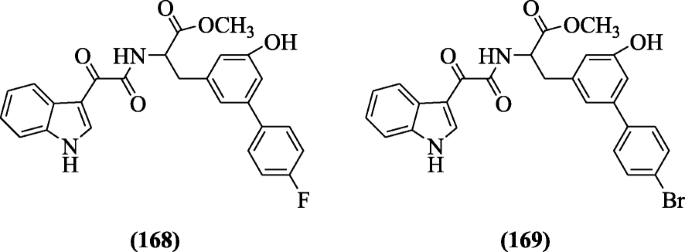
Indole-iii-glyoxyl tyrosine derivatives were reported past Vasconcelos et al. as antimalarial agents confronting the pathogen Plasmodium falciparum. Compounds (S)-methyl 2-(2-(1H-indol-three-yl)-2-oxoacetamido)-three-(4´-fluoro-6-hydroxy-[1, 1´-biphenyl]-3-yl)propenoate (168) and (S)-methyl 2-(2-(1H-indol-3-yl)-two-oxoacetamido)-3-(4´-bromo-6-hydroxy-[1,1´-biphenyl]-3-yl)propenoate (169) were favorable to antimalarial activity [83].
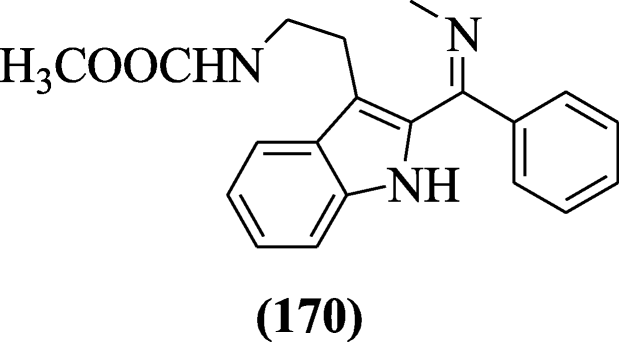
The potent antimalarial compounds were reported past Luthra et al. and chemical compound (Z)-methyl 2-(ii-((methylimino) (phenyl) methyl)-1H-indol-three-yl) ethylcarbamate (170) was active at the trophozoite stage of the parasite growth [84].
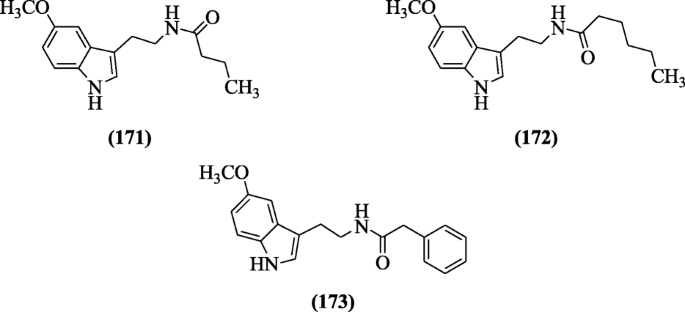
Schuck et al. synthesized melatonin compounds and assayed in Plasmodium falciparum culture for the measurement of antimalarial activities. Structural-activity relationship (SAR) showed that carboxamide group derivatives of indole gave a good result. Derivatives Due north-(2-(v-methoxy-1H-indol-3-yl)ethyl)butyramide (171), North-(2-(5-methoxy-1H-indol-3-yl)ethyl)hexanamide (172), and North-(2-(5-methoxy-1H-indol-3-yl)ethyl)-2-phenylacetamide (173) were active at low concentration against the Plasmodium falciparum [85].
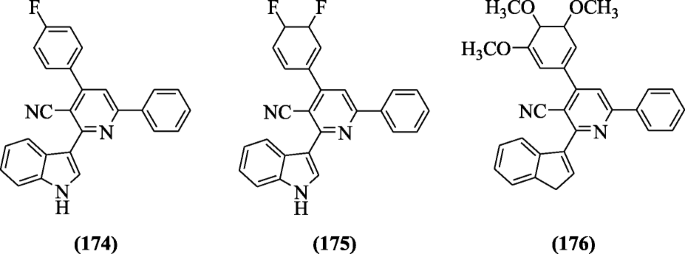
ii-(1H-indol-3-yl)-4, 6-diphenylnicotinonitrile derivatives containing pyridine were fabricated by Elshemy et al. and screened for antimalarial activity against Plasmodium falciparum. Among all the tested compounds, compounds 4-(4-fluorophenyl)-2-(aneH-indol-3-yl)-6-phenylnicotinonitrile (174), 4-(iii,four-difluorocyclohexa-1,five-dienyl)-2-(oneH-indol-3-yl)-6-phenylnicotinonitrile (175), and 2-(3H-inden-i-yl)-vi-phenyl-4-(three,iv,5-trimethoxycyclohexa-1,5-dienyl)nicotinonitrile (176) exhibit the highest selectivity alphabetize (S.I. ranged iii.viii–ten). Docking studies explained the interaction between compound and active site of the enzyme (quadruple mutant Plasmodium falciparum dihydrofolate reductase) [86]
gullettgocielince71.blogspot.com
Source: https://fjps.springeropen.com/articles/10.1186/s43094-020-00141-y